Preparation method of phenoxycarboxylic acid substances
A technology for phenoxycarboxylic acids and phenolic compounds is applied in the field of preparation of phenoxycarboxylic acids, and can solve the problems of complex technological process, complicated process and high consumption of sodium chlorocarboxylate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a kind of preparation method of phenoxycarboxylic acid substances, comprising:
[0028] S1) mixing the phenolic compound represented by formula (I), the halogenated fatty alcohol represented by formula (II) and alkali metal carbonate in an organic solvent, heating and reacting to obtain phenoxy fatty alcohol;
[0029] S2) reacting the phenoxy fatty alcohol with an oxidizing agent to obtain phenoxy fatty acid;
[0030] S3) Mixing and reacting the phenoxy fatty acid, the chlorination catalyst and the chlorination agent to obtain the phenoxycarboxylic acid substances represented by the formula (III);
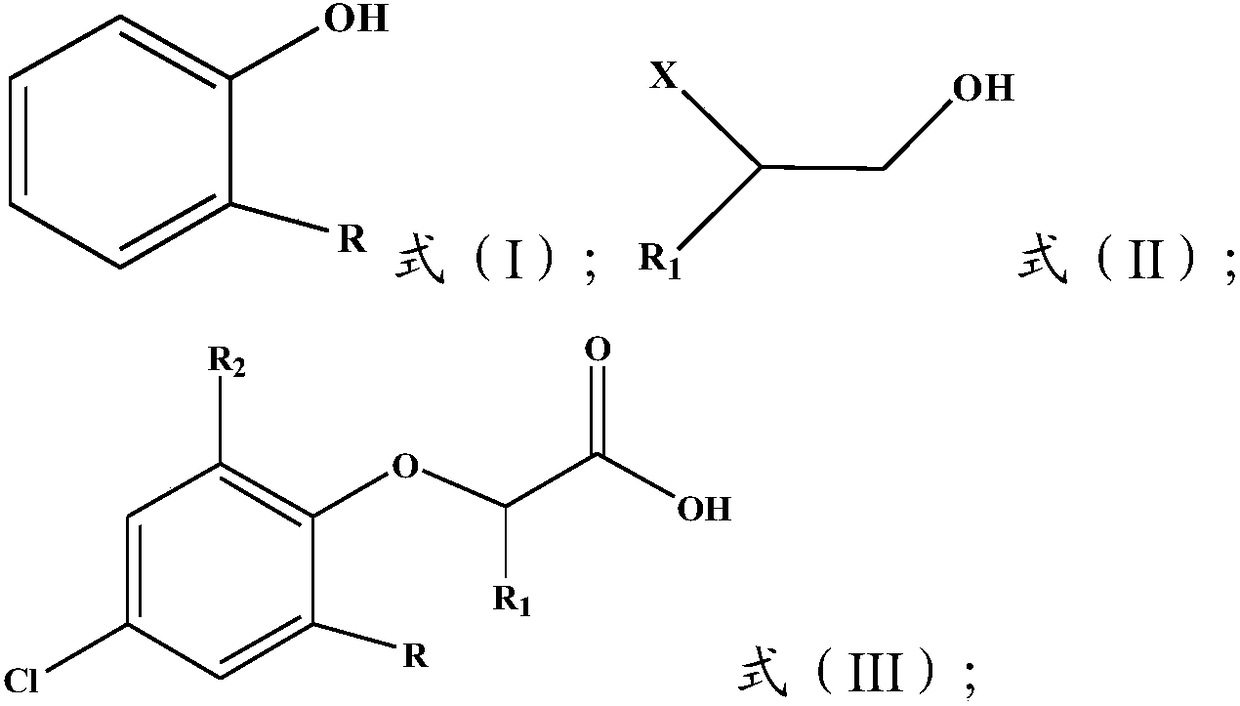
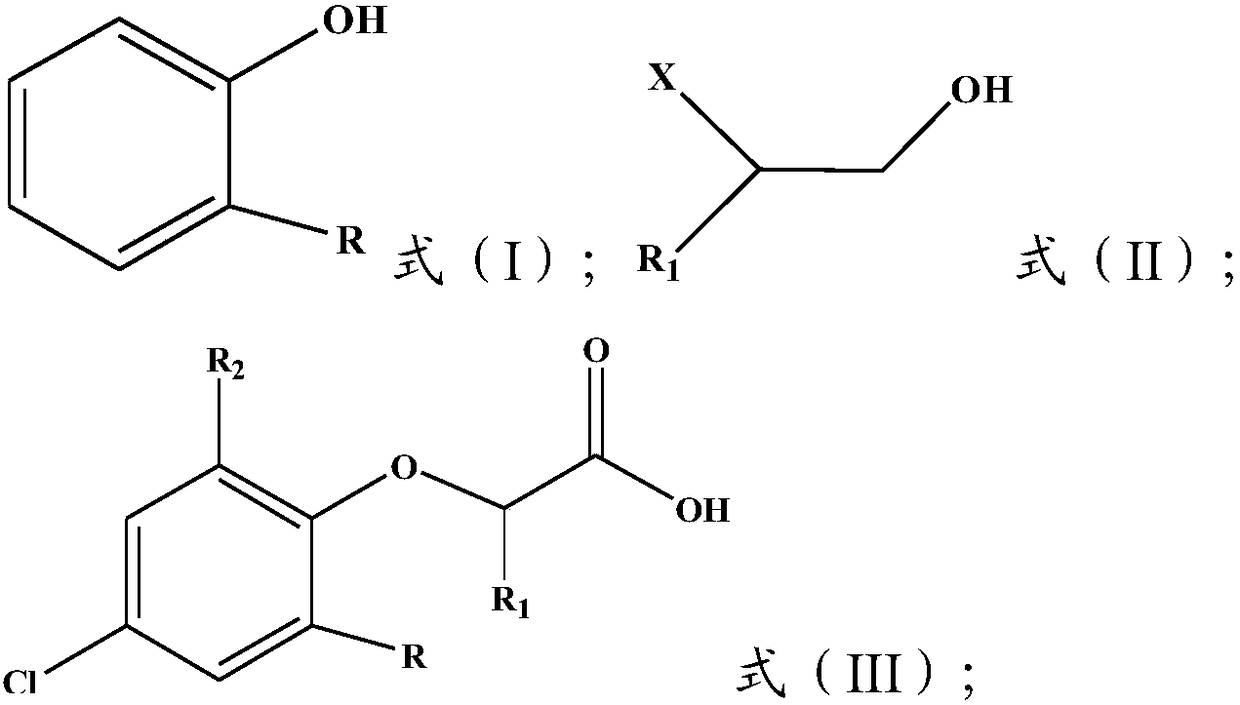
[0031]
[0032] Wherein, R is H or C1~C5 alkyl, preferably C1~C3 alkyl, more preferably C1~C2 alkyl, more preferably methyl; R 1 It is H or C1-C4 alkyl, preferably H or C1-C3 alkyl, more preferably H or C1-C2 alkyl; R 2 is Cl or H, and R and R 2 Not simultaneously H; X is halogen, preferably Cl.
[0033] The present invention has no special limi...
Embodiment 1
[0044] Add 97g 98.5% phenol, 81g 99% chloroethanol, 145g 99% potassium carbonate and 300g DMF into the reactor, raise the temperature to 130°C, keep the temperature for 6 hours, cool down to room temperature, then filter to remove the salt, add fresh DMF Wash the filter cake twice, then mix the filtrate to obtain phenoxyethanol after rectification treatment, then add 139.6g (1mol) phenoxyethanol, water 300g, add 14g activated carbon, add 350g 10% hydrogen peroxide, heat up to about 120°C and keep 12 Hours, check the content of phenoxyethanol, when the content of phenoxyethanol is less than 0.1%, cool down to about 80°C, add 1.4g of formamide, and pass in 144g of chlorine gas, the aeration time is controlled at 2 to 5 hours. After the chlorination is completed, cool down , filtered, and dried to obtain 214.1 g of 2,4-dichlorophenoxyacetic acid with a purity of 98.2% and a yield of 95.1%.
Embodiment 2
[0046] Add 97g 98.5% phenol, 81g 99% chloroethanol, 145g 99% potassium carbonate, and 300g DMSO into the reactor, raise the temperature to 150°C, keep it warm for 5 hours, cool down to room temperature, then filter to remove salt, add fresh DMSO to wash Filter the cake twice, then mix the filtrate to obtain phenoxyethanol after rectification treatment, add 139.6g (1mol) of phenoxyethanol, 300g of water, add 28g of white carbon black, pass in 32g of ozone, heat up to about 120°C and keep for 12 hours, Check the phenoxyethanol content, when the phenoxyethanol content is less than 0.1%, wait until it is not qualified. Cool down to about 80°C, add 1.5g of formamide, add dropwise 270g of sulfuryl chloride, and control the dropwise addition time within 2 to 5 hours. After the chlorination is completed, cool down, filter, and dry to obtain 2,4-dichlorophenoxyacetic acid 217.4 g, purity 98.1%, yield 96.6%.
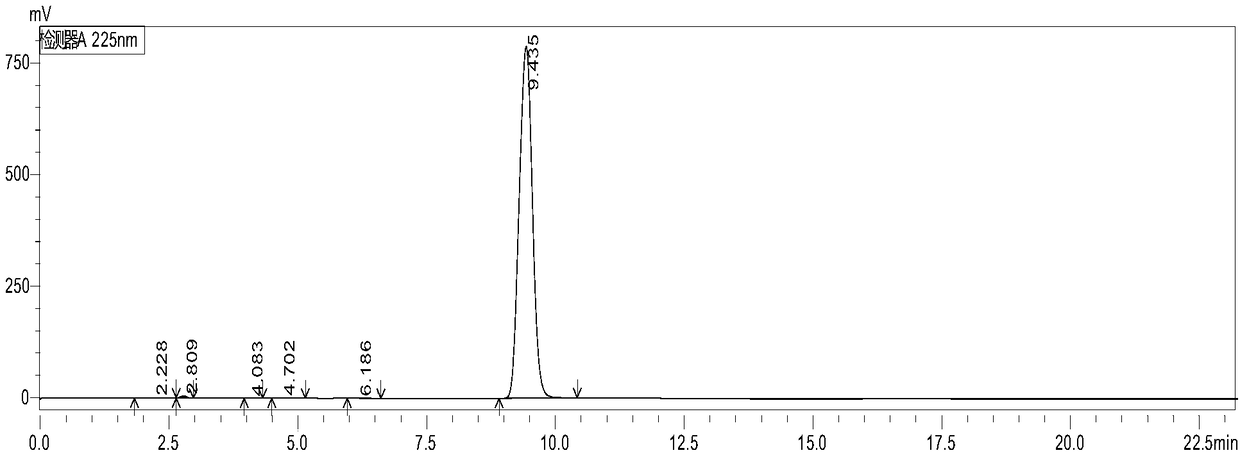
[0047] Utilize high performance liquid chromatography to analyze the 2,4-dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com