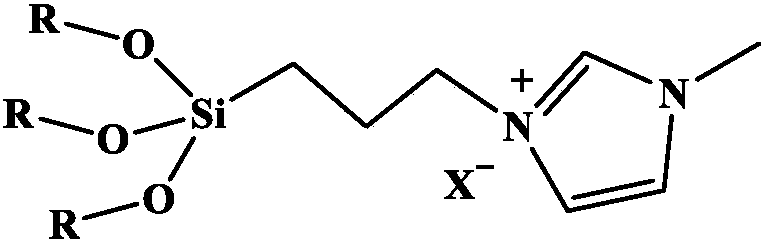Catalysis system for synthesizing methyl propionate and application method thereof
A catalytic system, a technology of methyl propionate, applied in catalytic reactions, chemical instruments and methods, carbon monoxide or formate reaction preparation, etc., can solve the problems of high production cost, harsh reaction conditions, easy decomposition, etc., and achieve recycling good performance, improve catalytic activity and shorten the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
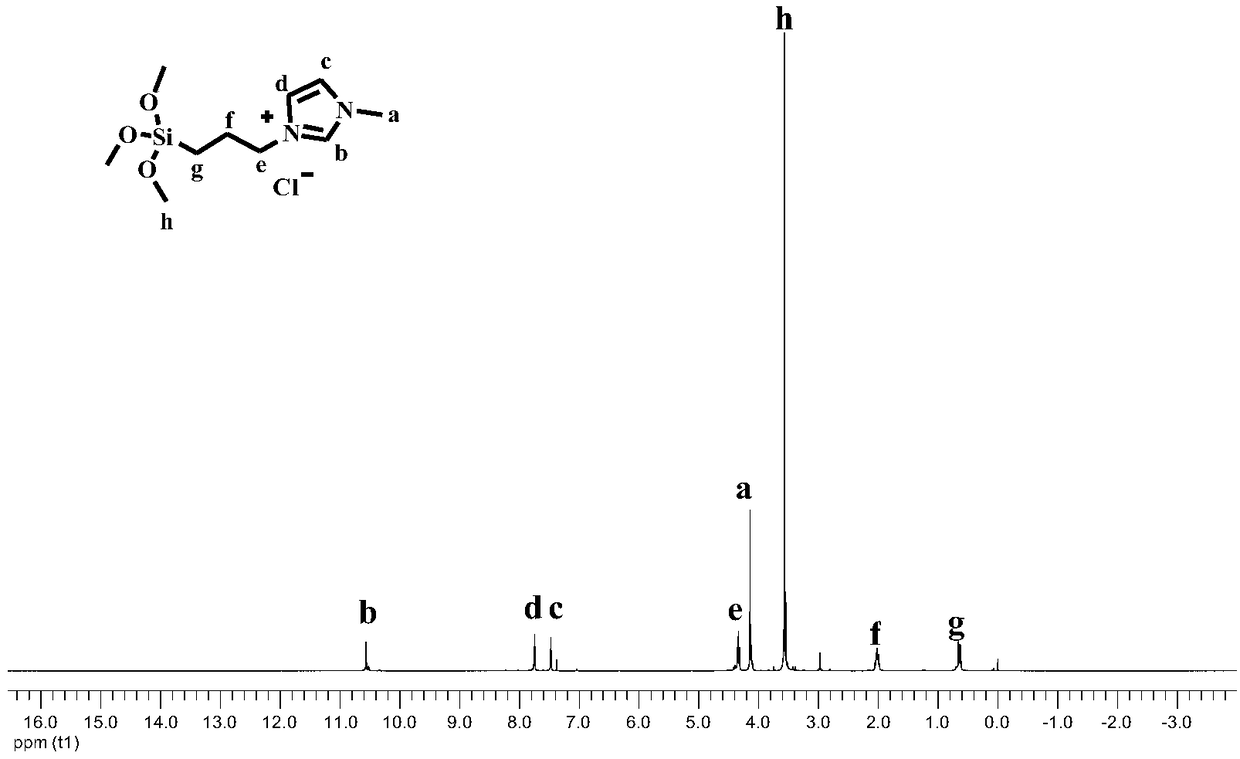
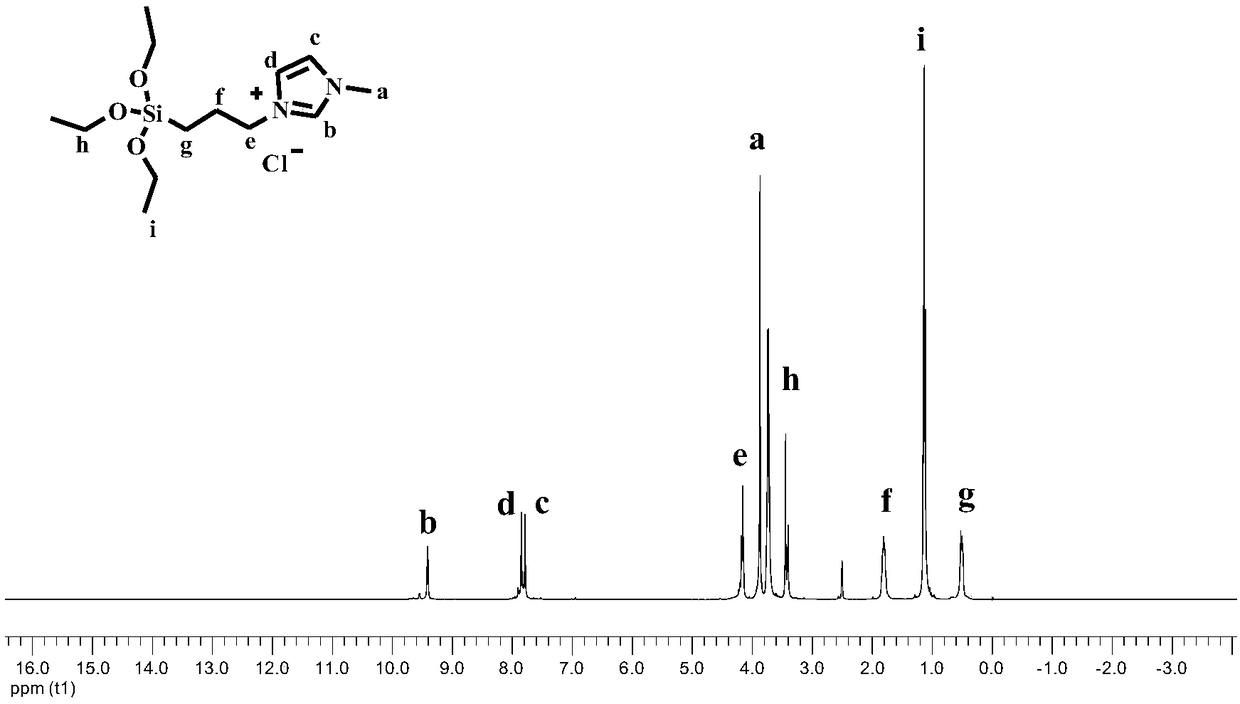
[0042] Weigh Ionic liquid (1) 5.6mmol, Ru 3 (CO) 12 Add 0.042 mmol into the reaction kettle, seal the reaction kettle, put the reaction kettle into an oil bath at a temperature of 70°C, and vacuumize for 0.5h. Add 5.6ml (150mmol) of methanol and 2mmol of ethylene into the reaction kettle under the protection of argon, and fill with CO 2 4MPa and equilibrate the pressure for 3min, the temperature of the reactor was raised to 200°C, the stirring speed was 500r / min, and the reaction lasted for 6h. After the reaction, the liquid in the kettle was orange-red transparent liquid, and the yield of methyl propionate was 98.9%. The test method of the yield: remove the transparent liquid in the reaction kettle into a 10ml vial, add a certain mass of n-heptane into the vial as an internal standard, record the mass of n-heptane added, and calculate the amount of n-heptane by the internal standard method of gas chromatography. The yield of methyl esters.
Embodiment 2
[0044] Others are the same as in Example 1, except that the reaction temperature is 160° C., the reaction time is 20 h, and the yield of methyl propionate is 99.7%. Under the same conditions in the literature, the synthetic yield of methyl propionate is 76.0%.
Embodiment 3
[0046] Others are the same as in Example 1, except that the Ionic liquid (1) is replaced by the ionic liquid [Bmim]Cl in the literature, and the yield of methyl propionate is 37.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com