Preparation method of recombinant human insulin
A technology for recombining human insulin and proinsulin, applied in insulin, recombinant DNA technology, chemical instruments and methods, etc., can solve the problems of amino acid residues at enzyme cleavage sites, poor stability, and low cleavage efficiency, and achieve a simple and convenient conversion process. The effect of simplifying the technological process and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Design of Sumo-proinsulin fusion gene
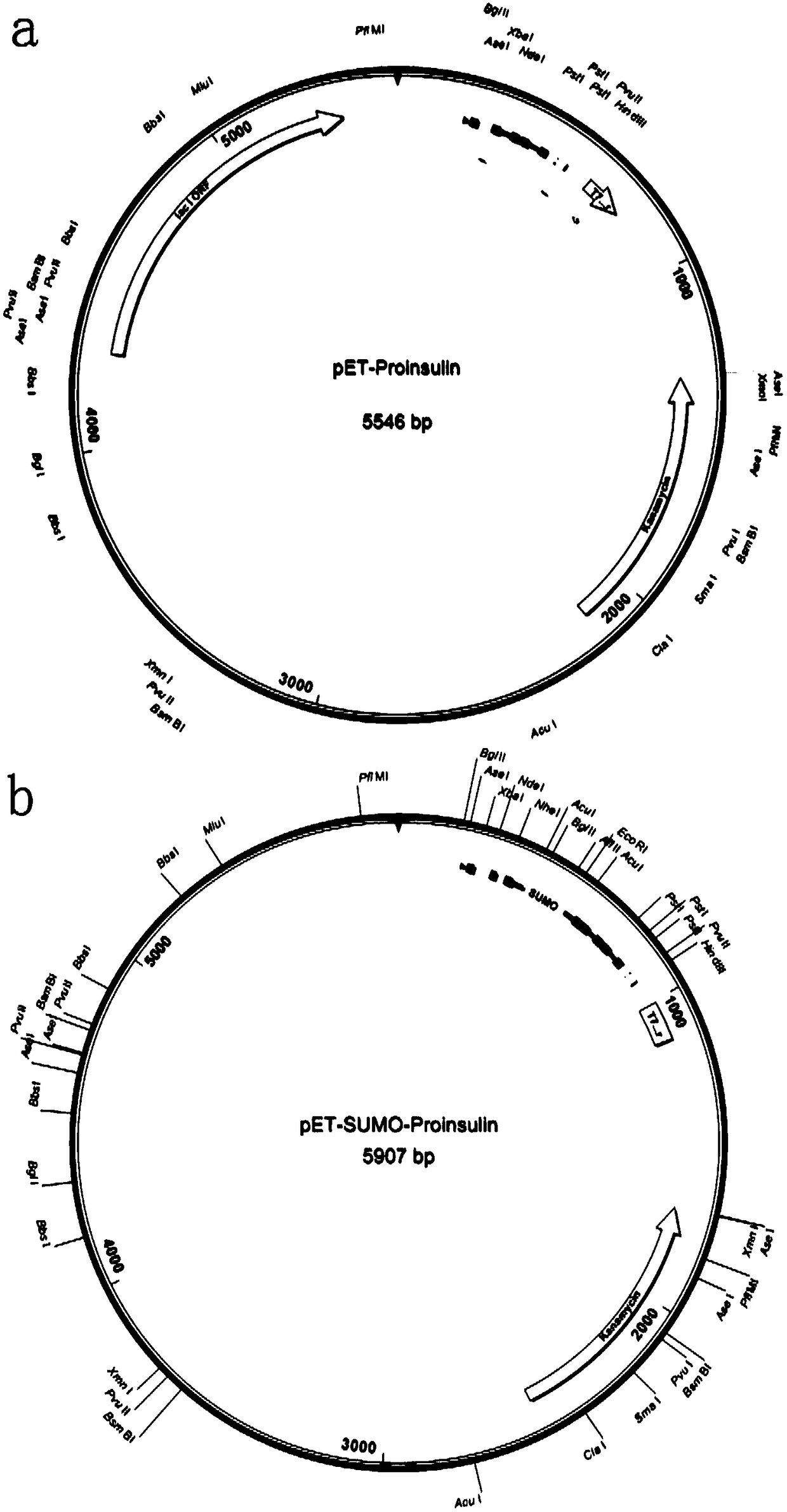
[0043] Using pET-SUMO as the basic vector, design and construct the pET-SUMO-Proinsulin recombinant plasmid with Sumo tag ( figure 1 a) The plasmid includes the T7 promoter, Lac controller, proinsulin gene and Sumo gene coding region (SEQ ID No. 1).
[0044] In order to test the effect of the Sumo fusion tag on the expression of proinsulin in E. coli, a pET-Proinsulin plasmid (proinsulin) without the Sumo tag was constructed ( figure 1 b) The plasmid includes T7 promoter, Lac controller, and proinsulin gene coding region (SEQ ID No. 3). The specific method is as follows:
[0045] A DNA fragment with a full length of 357 bases (SEQ ID No. 2) was obtained by chemical synthesis. This fragment has EcoRI and HindIII endonuclease sites at the 5'and 3'ends, respectively. The middle sequence contains the 28 amino acids at the C-terminal of the compiled Sumo and the full length of human insulin (A chain + B chain + C peptide ) DNA seq...
Embodiment 2
[0046] Example 2 Induced expression of recombinant Sumo-proinsulin protein
[0047] The constructed recombinant plasmids of pET-Proinsulin and pET-SUMO-Proinsulin, which have been constructed and confirmed by DNA sequencing, were transferred into BL21(DE3) cells by traditional heat shock method, and single clones were selected and grown in LB medium at 37°C To OD 600 =0.5, then add 1mM IPTG to induce expression for 6 hours.
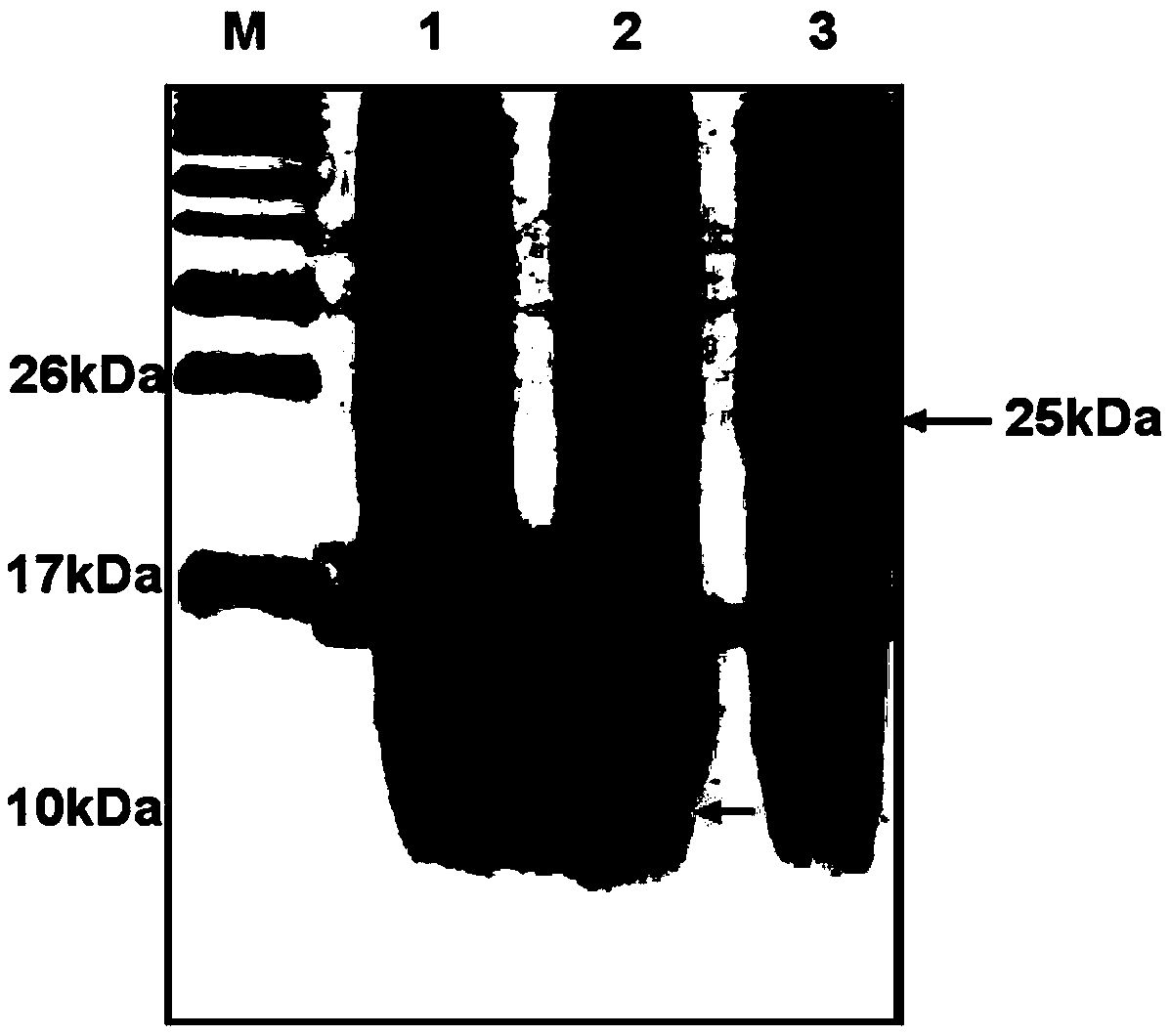
[0048] In order to compare the effect of Sumo fusion tag on the expression of proinsulin protein in E. coli, after the induction, the bacterial solution was centrifuged and the obtained large intestine was lysed, and then subjected to gel electrophoresis analysis. The results are shown in figure 2 . figure 2 In the middle, lane M represents the marker; lane 1 represents Pet-Sumo (positive control), with a relative molecular mass of 17kDa; lane 2 represents Pet-Insulin (C peptide), with a theoretical relative molecular mass of 9kDa, and the arrow indicates an ...
Embodiment 3
[0049] Example 3 Denaturation and renaturation of recombinant Sumo-proinsulin protein
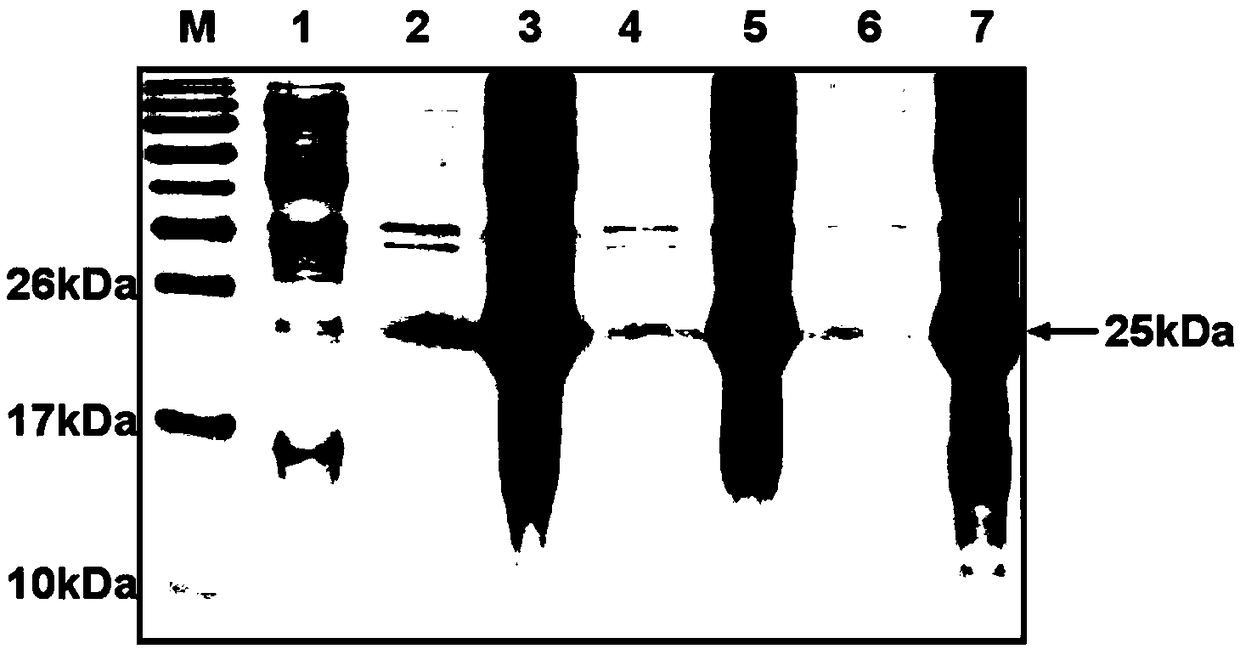
[0050] After the induction in Example 2, the Escherichia coli expressing the recombinant Sumo-proinsulin protein was lysed, and the precipitate was collected to obtain insoluble inclusion bodies. At the same time, the supernatant and precipitate were analyzed by polyacrylamide gel electrophoresis, the results are as follows image 3 Shown. image 3 Lane M indicates the marker; lane 1 indicates the uninduced E. coli lysate after transfer to the plasmid; lane 2 indicates the supernatant after the first wash with 1×PBS; lane 3 indicates the first time with 1×PBS Precipitation after washing; Lane 4 represents the supernatant after the second wash with 1×PBS; Lane 5 represents the precipitation after the second wash with 1×PBS; Lane 6 represents the supernatant after washing with washing solution; Lane 7 represents the supernatant after washing with washing solution precipitation. image 3 The resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com