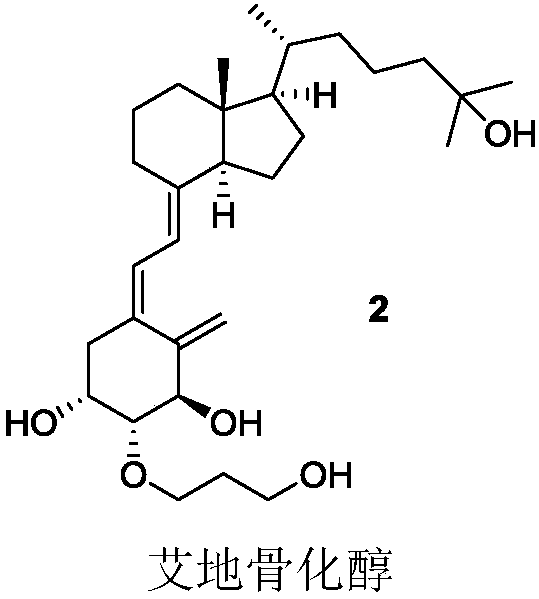
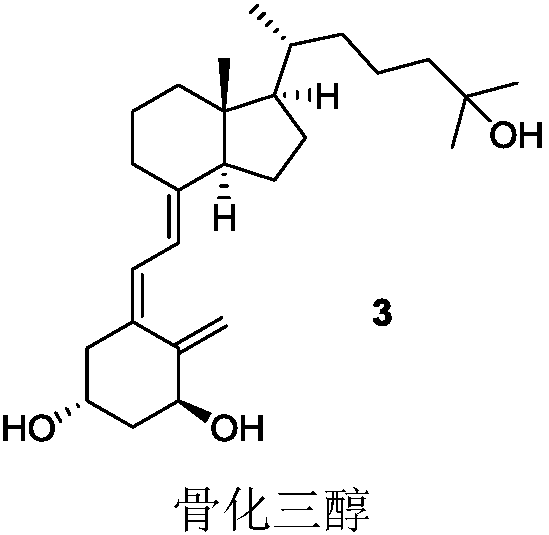
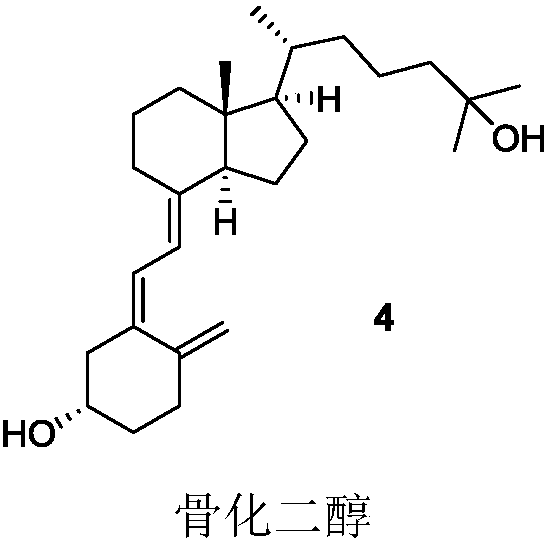
Cyclic cholane carboxylate derivative, preparation method and uses thereof
A technology for cyclocholane carboxylate and derivatives, which is applied in the field of industrial preparation of 25-hydroxycholesterol, and can solve the problem of unclear sources of 24-dehydrocholesterol derivatives, unmarked chiral carbon stereo configuration, and the need for additional preparations, etc. problems, to achieve the effects of reliable commercial sources, favorable industrial production, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, the preparation of (3β, 5α, 6β)-6-methoxy-3,5-cyclocholane-24-carboxylic acid methyl ester (Ia)
[0054]
[0055] Nickel chloride hexahydrate (0.6g, 2.5mmol), methyl acrylate (0.86g, 10.0mmol) and pyridine (4.5ml) were placed in a reaction flask, and zinc powder (0.7g, 10.0mmol) was added under stirring; Under nitrogen protection, heat to 65°C and stir for 30min. After cooling to room temperature, a solution of Va (1.0 g, 2.2 mmol) in pyridine (4.5 ml) was added dropwise, then stirred at room temperature for 2.5 h. Filter under diatomaceous earth filter, add ethyl acetate (50ml) to the filtrate, wash with 0.5mol / L sulfuric acid aqueous solution to pH 2~3, then use ethylenediaminetetraacetic acid (EDTA) aqueous solution (preparation method: EDTA (80g) and sodium bicarbonate (80g) were dissolved in water (1L) (40ml), washed with saturated brine (40ml), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting residue wa...
Embodiment 2
[0056] Embodiment 2, the preparation of (3β, 5α, 6β)-6-methoxy-3,5-cyclocholane-24-carboxylic acid ethyl ester (Ib)
[0057]
[0058] Nickel chloride hexahydrate (1.0g, 4.2mmol), ethyl acrylate (2.23g, 22mmol) and pyridine (9ml) were placed in a reaction flask, and zinc powder (1.3g, 20mmol) was added under stirring; , heated to 65°C and stirred for 30min. After cooling to room temperature, a solution of Va (2.0 g, 4.4 mmol) in pyridine (9 ml) was added dropwise, then stirred at room temperature for 2.5 h. Filtrate under diatomite filter, add ethyl acetate (50ml) to the filtrate, wash with 0.5mol / L sulfuric acid aqueous solution to pH 2~3, then wash with EDTA aqueous solution (50ml) and saturated brine (50ml) successively, Dry over anhydrous sodium sulfate and concentrate under reduced pressure. The resulting residue was purified by flash preparative chromatography (eluent: ethyl acetate-n-hexane, gradient elution: ethyl acetate 1% to 2%), and dried in vacuo to give a col...
Embodiment 3
[0059] Embodiment 3, the preparation of (3β, 5α, 6β)-6-methoxyl-3,5-cyclocholane-24-carboxylic acid propyl ester (Ic)
[0060]
[0061] Nickel chloride hexahydrate (0.6g, 2.5mmol), propyl acrylate (1.1g, 9.6mmol) and pyridine (4.5ml) were placed in a reaction flask, and zinc powder (0.7g, 10.0mmol) was added under stirring; Under nitrogen protection, heat to 65°C and stir for 30min. After cooling to room temperature, a solution of Va (1.0 g, 2.2 mmol) in pyridine (4.5 ml) was added dropwise, then stirred at room temperature for 2.5 h. Filtrate under diatomite filter, add ethyl acetate (30ml) to the filtrate, wash with 0.5mol / L sulfuric acid aqueous solution to pH 2~3, then wash with EDTA aqueous solution (30ml) and saturated brine (30ml) successively, Dry over anhydrous sodium sulfate and concentrate under reduced pressure. The resulting residue was purified by flash preparative chromatography (eluent: ethyl acetate-n-hexane, gradient elution: ethyl acetate 1% to 2%), and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com