Hydroxylation method of recombinant human-derived collagen
A collagen and hydroxylation technology, applied in chemical instruments and methods, animal/human proteins, biochemical equipment and methods, etc., can solve the problem of high expression of collagen produced by recombinant bacteria, harsh exogenous expression conditions, and large-scale application problems such as suitable for large-scale production, easy high-throughput expression, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] According to the above method, the present invention also relates to a method for preparing hydroxylated recombinant human-derived collagen, comprising the following steps:
[0044] (1) Construct a co-expression strain of the human collagen gene and the proline hydroxylase gene derived from Cystis spp. RH1, the gene sequence is SEQ ID No.1, and the corresponding amino acid sequence is SEQ ID No.2;
[0045] (2) Express collagen and proline hydroxylase;
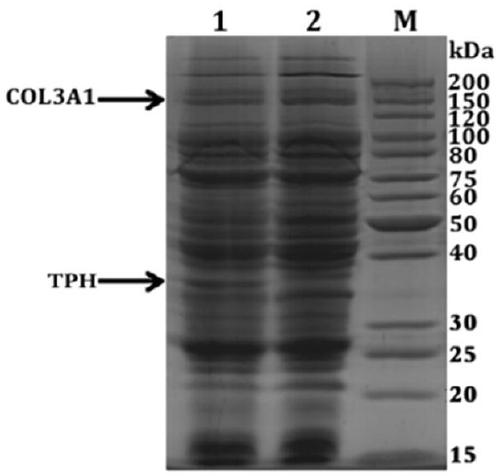
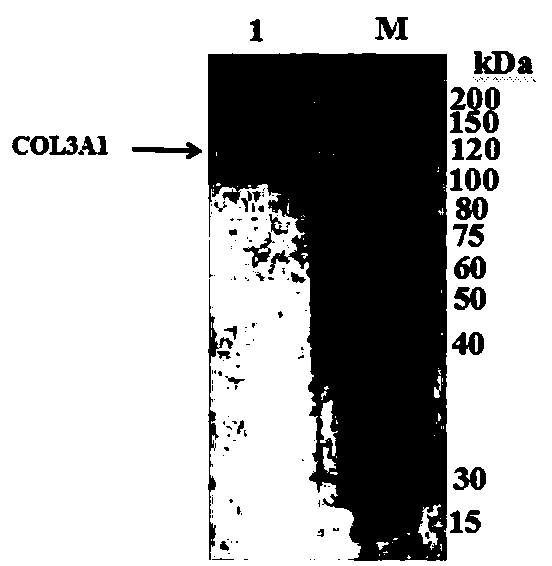
[0046] (3) Separation and purification of collagen to obtain hydroxylated recombinant collagen.
[0047] Step (1) is specifically:
[0048] (1.1) Obtain the collagen gene and the proline hydroxylase gene derived from the cystic fungus RH1 by means of PCR or whole gene synthesis;
[0049] (1.2) Select the appropriate expression system and construct the corresponding plasmid;
[0050] (1.3) Transfer the plasmid into the expression host;
[0051] (1.4) Screen positive transformants to obtain co-expression strains.
[0...
Embodiment 1
[0058] Example 1: Preparation of Hydroxylated Recombinant Human Type III Collagen Alpha Chain Protein
[0059] 1. Co-expression strain construction
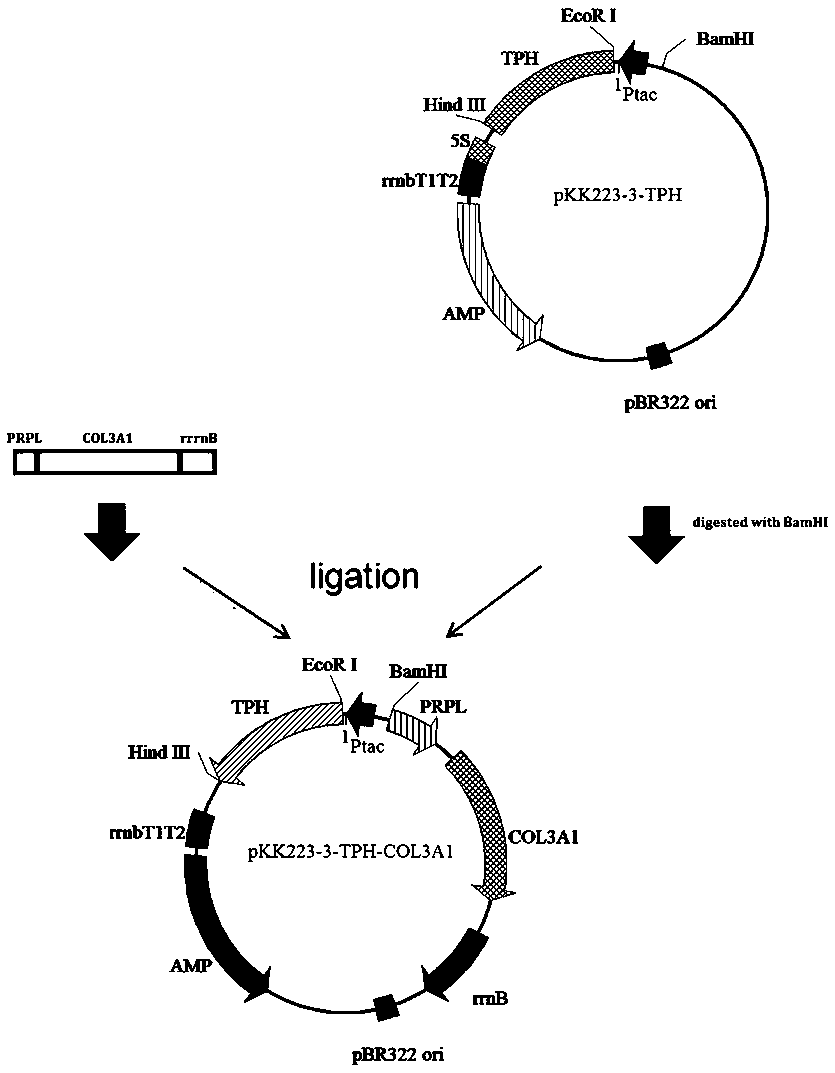
[0060] 1) p Construction of KK223-3-TPH: the proline hydroxylase gene (TPH) derived from the cystic fungus RH1, the gene sequence is SEQ ID No.1, and the corresponding amino acid sequence is SEQ ID No.2 through whole gene synthesis After synthesis, it is directly connected to the pKK223-3 plasmid p KK223-3-TPH.
[0061] ) p Construction of BV220-COL3A1: Total RNA was extracted from fresh human placenta tissue, and a cDNA library was established by reverse transcription. Using the cDNA library as a template, the human type III collagen α chain gene CO3A1 was amplified by PCR with the following primers (GenBank: AAH28178.1 ), the PCR product was double digested and ligated into the vector p BV220 was transformed into Escherichia coli and the positive transformant was picked to extract the plasmid. p BV220-COL3A1. The PCR pri...
Embodiment 2
[0078] Example 2 Hydroxylated recombinant human Collagen type α chain protein preparation
[0079] 1. Co-expression strain construction
[0080] 1) p PICZB-TPH build: with p The KK223-3-TPH plasmid was used as a template to amplify the TPH gene and connect it with the EcoRI restriction site p Among the PICZB plasmids, positive clones were screened, and the plasmids were extracted. p PICZB-TPH plasmid.
[0081] ) p PIC9k-COL1A1 construction: total RNA was extracted from fresh human placenta tissue, a cDNA library was established by reverse transcription, and the cDNA library was used as a template to amplify human Collagen type α chain gene COL1A1 (Gene ID: 1277), the PCR product was double digested and ligated into the vector p PIC9k was transformed into Escherichia coli and the positive transformants were picked to extract the plasmid. p PIC9k-COL1A1.
[0082] ) Preparation of co-expression strains
[0083] Will p PICZB-TPH and p PIC9k-COL1A1 was linearized wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com