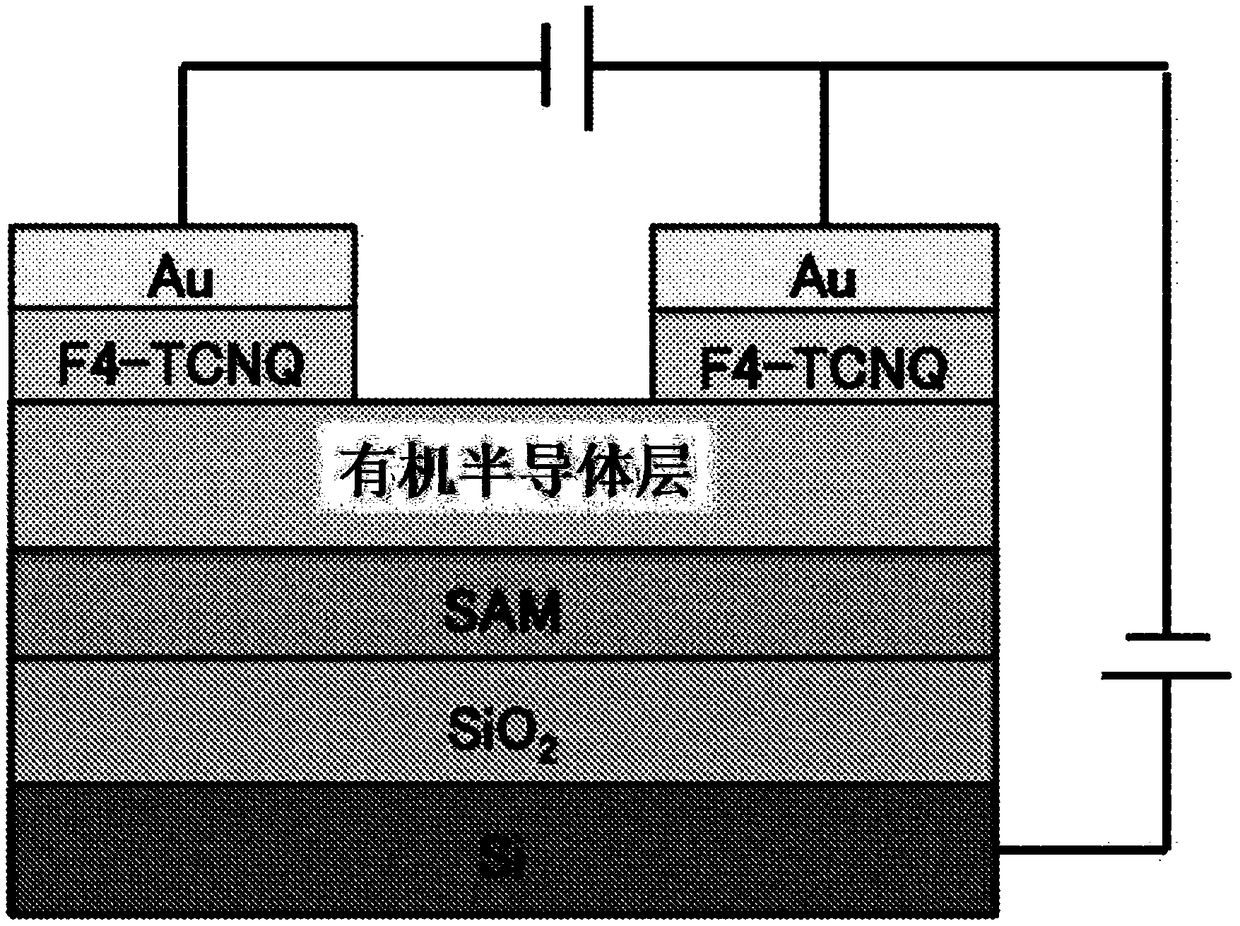
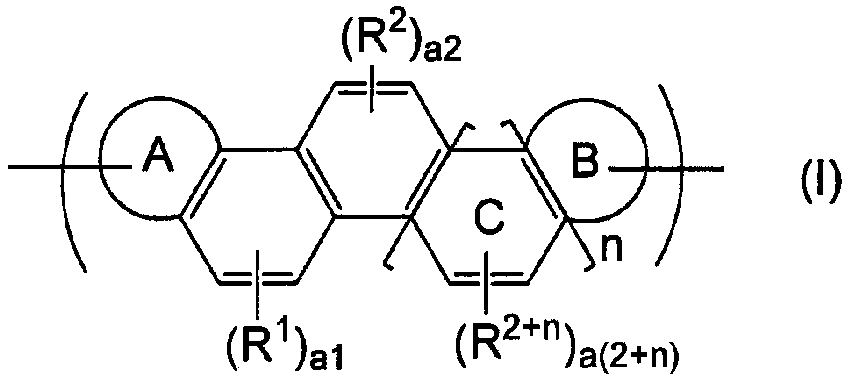
Novel organic polymer and method for producing same
A technology of organic polymers and organic semiconductors, which is applied in the fields of final product manufacturing, sustainable manufacturing/processing, semiconductor/solid-state device manufacturing, etc. It can solve the problems of insufficient carrier mobility and low film strength, and achieve a large overlapping range, Effect of improving solubility and high carrier mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0199] Hereinafter, the present invention will be described in more detail based on examples, but the present invention is not limited to these examples.
Synthetic example 1
[0201]
[0202] An orange suspension of 2,6-dibromonaphthalene-1,5-diol (1) (100 g, 315 mmol) / dichloromethane (700 mL) / pyridine (101 mL, 126 mmol) was cooled to 0 °C under argon atmosphere . While stirring, trifluoromethanesulfonic anhydride (2) (134 mL, 818 mmol) / dichloromethane (100 mL) solution was added dropwise, and the dark red suspension was stirred at room temperature for 1 hour. Water was added to the reaction mixture, and the organic layer was extracted with chloroform. After the extract was filtered through silica gel, the filtrate was concentrated under reduced pressure, and the resulting crude product was washed with acetone to obtain the target compound (3) (2,6-dibromonaphthalene-1,5-bis Triflate) as a white solid (110 g, 60% yield).
[0203] NMR: 1H-NMR (400MHZ, CDCl3): δ (PPm) 7.88 (d, 2H, J = 8.8HZ, ARH), 8.02 (d, 2H, J = 8.8HZ, ARH).
Synthetic example 2
[0205]
[0206]Under argon atmosphere, argon was bubbled into a brown solution of compound (3) (1 g, 1.7 mmol) / N,N-dimethylformamide (7 mL) / diisopropylamine (8.6 mL) for 15 minutes. Thereafter, while stirring at room temperature, copper(I) iodide (32.7 mg, 0.17 mmol), [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) After methyl chloride complex (70.2 mg, 0.086 mmol), trimethylsilylacetylene (TMS-≡) (4) (0.5 mL, 3.6 mmol), the black suspension was stirred at room temperature for 12 hours. After diluting the reaction mixture with chloroform, it filtered through silica gel. The filtrate was concentrated under reduced pressure, and the resulting crude product was recrystallized in hexane to obtain the target compound (5) (2,6-dibromo-1,5-bis(2-trimethylsilane ethynyl)naphthalene) as a pale yellow solid (0.79 g, 86% yield).
[0207] NMR: 1H-NMR (400MHZ, CDCl3): δ (PPm) 0.35 (S, 18H, Si (CH3) 3), 7.71 (d, 2H, J = 8.8HZ, ARH), 8.14 (d, 2H, J = 8.8HZ, ARH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com