Method of extracting bromine from bromine-bearing liquids or wastewaters
A technology for feed liquid and waste water, applied in the direction of bromine/hydrogen bromide, bromine, chlorine/hydrogen chloride, etc., can solve the problems that hinder the popularization and application of solvent extraction method, it is difficult to obtain high-purity products, and it takes a long time for phase equilibrium to achieve extraction The effect of small dosage, high saturation capacity and fast establishment time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
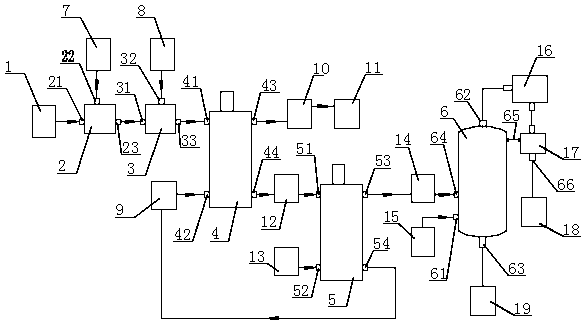
[0028] Realize that the used bromine extracting device of process method of the present invention is as figure 1As shown, it includes a raw material tank 1, an acidification tank 2, an oxidation tank 3, a first centrifugal extractor 4, a second centrifugal extractor 5 and a distillation tower 6 arranged in sequence according to the process route; the acidification tank 2 is provided with a feed liquid inlet 21. An acidifying agent inlet 22 and an acidifying liquid outlet 23; the oxidation tank 3 is provided with an acidizing liquid inlet 31, an oxidizing agent inlet 32 and an oxidizing liquid outlet 33; the raw material tank 1 communicates with the feed liquid inlet 21, and the acidifying agent inlet 22 It communicates with the acidifying agent supply device 7 , the acidizing liquid outlet 23 communicates with the acidizing liquid inlet 31 , and the oxidant inlet 32 communicates with the oxidant supply device 8 . The first centrifugal extractor 4 has a first upper liquid i...
Embodiment 2
[0031] The method for extracting bromine comprises the following steps:
[0032] (1) The salt-making bittern in the raw material tank 1 enters the acidification tank 2 through the pipeline, and at the same time, the concentrated hydrochloric acid is sent to the acidification tank 2 through the acidifier supply device 7, and the salt-making bittern is acidified into a pH value of 3.0-4.0 Acidification solution: the acidification solution enters the oxidation tank 3 through the pipeline, and at the same time, chlorine gas is introduced into the oxidation tank 3 through the oxidant supply device 8, and the bromide ions in the acidification solution are oxidized into free bromine, thereby obtaining the oxidation solution, the bromine of the oxidation solution The element content is 0.68g / L. The amount of chlorine introduced in moles is 1.1 times the molar number of bromide ions in bittern for salt making.
[0033] (2) The oxidizing liquid in the oxidation tank 3 flows into the fi...
Embodiment 3
[0037] The method for extracting bromine comprises the following steps:
[0038] (1) The bromine-containing phosphate ore tail water in the raw material tank 1 enters the acidification tank 2 through the pipeline, and at the same time, the concentrated hydrochloric acid is sent to the acidification tank 2 through the acidifier supply device 7 to acidify the phosphate ore tail water to a pH value of 2.0 -3.0 acidizing solution; the acidizing solution enters the oxidation tank 3 through the pipeline, and simultaneously feeds chlorine gas into the oxidation tank 3 through the oxidant supply device 8 to oxidize the bromide ions in the acidizing solution into free bromine, thereby preparing the oxidation solution. The bromine content of the liquid is 1.35g / L. The amount of chlorine introduced in moles is 1.3 times the molar number of bromide ions in the salt-making bittern.
[0039] (2) The oxidizing liquid in the oxidation tank 3 flows into the first centrifugal extractor 4 throu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com