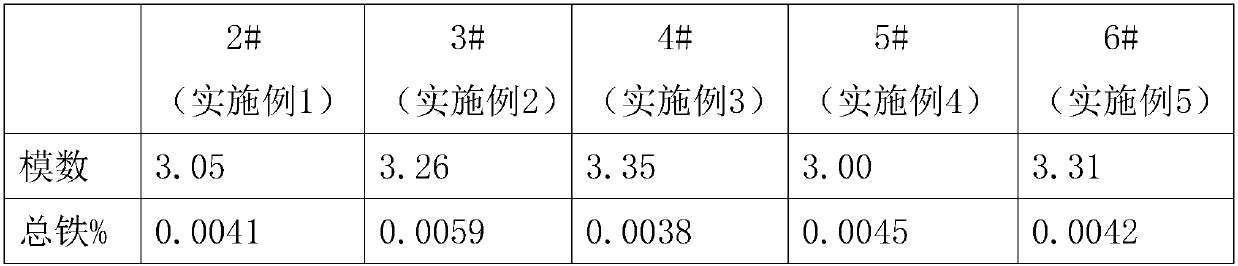
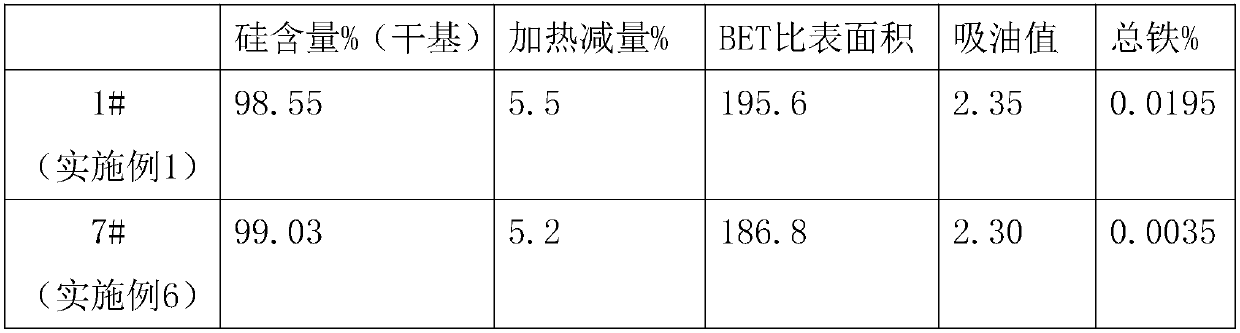
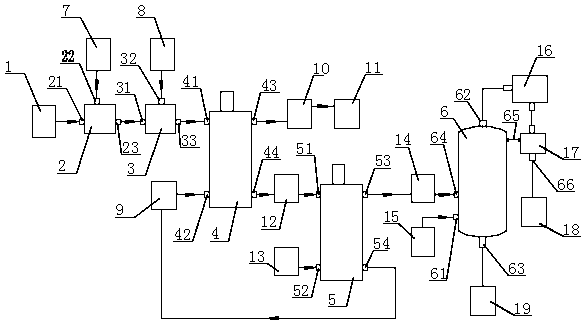
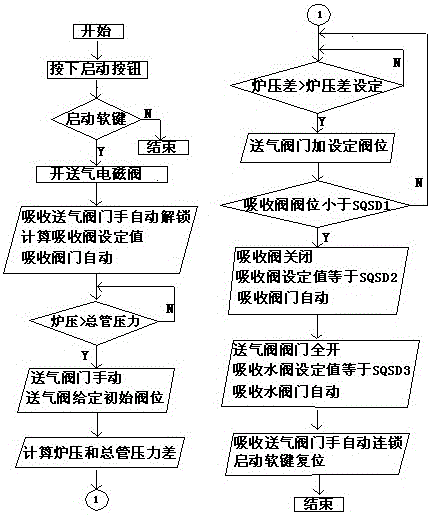
Patents
Literature
155results about "Hydrogen chloride preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
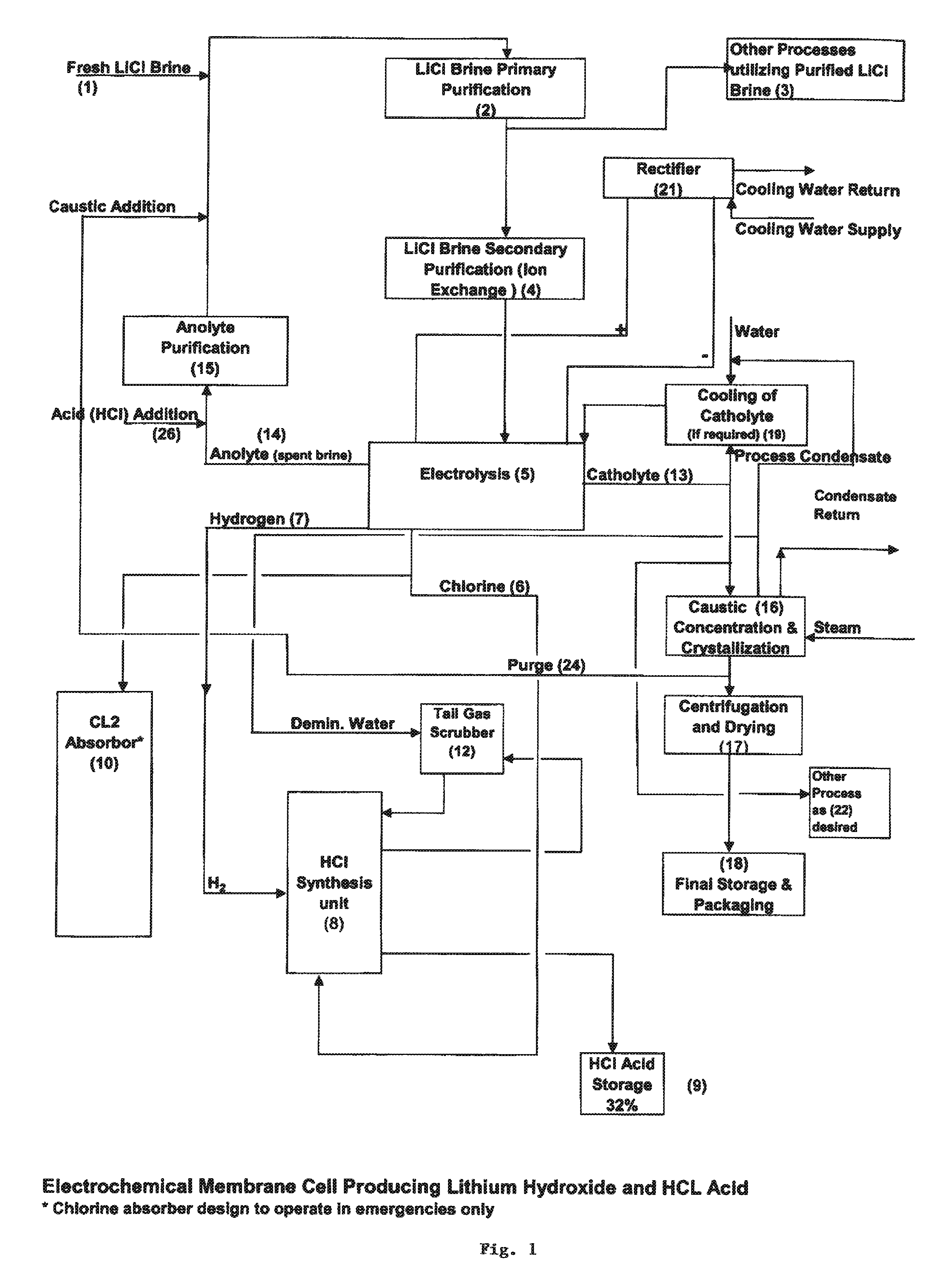
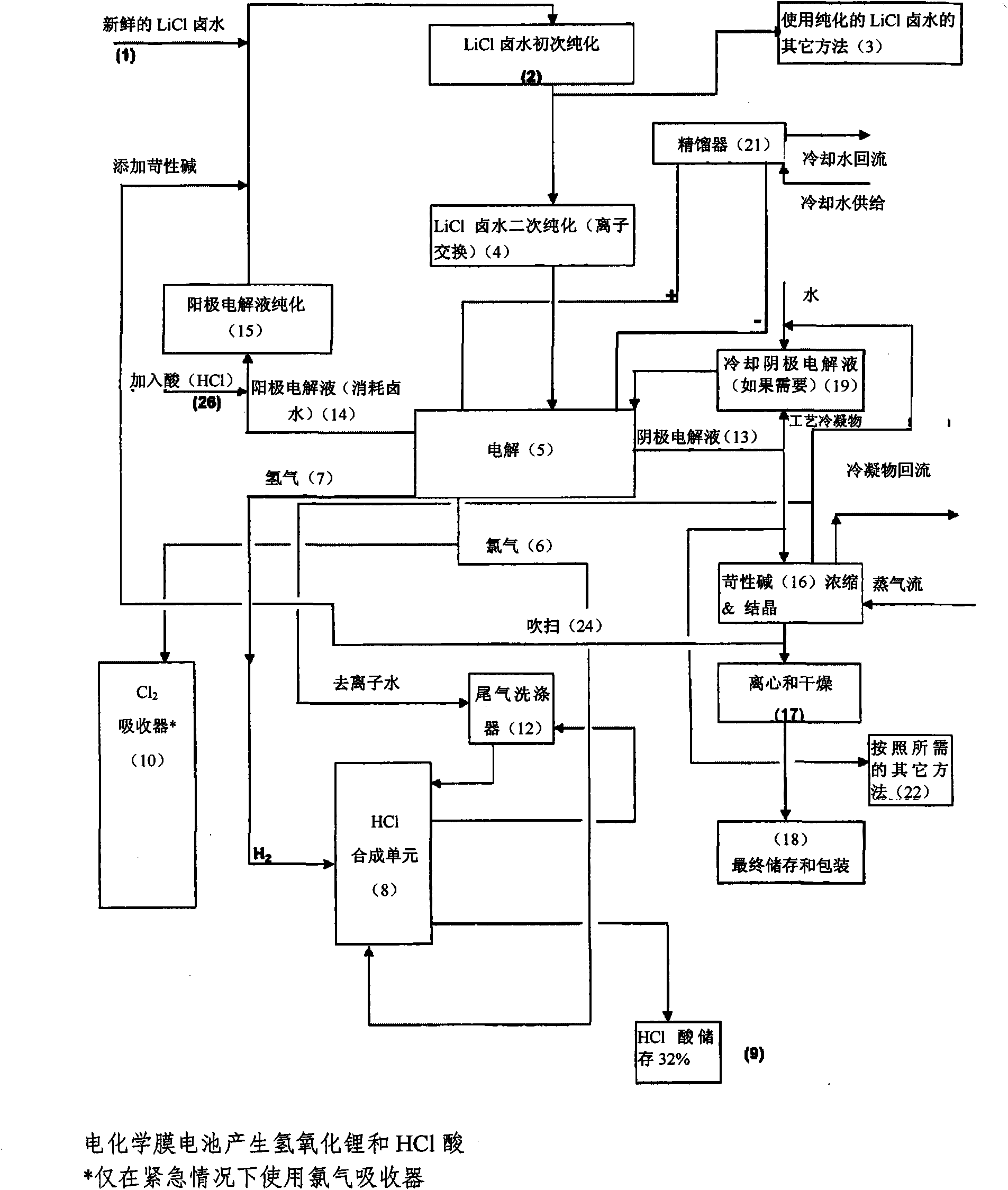
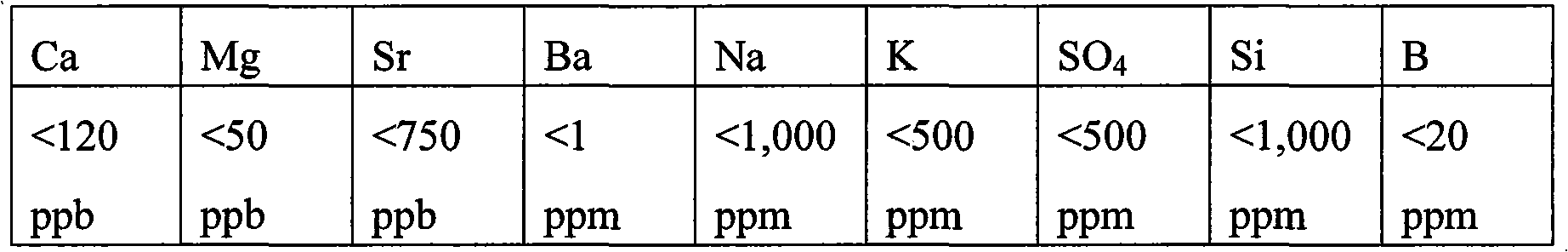
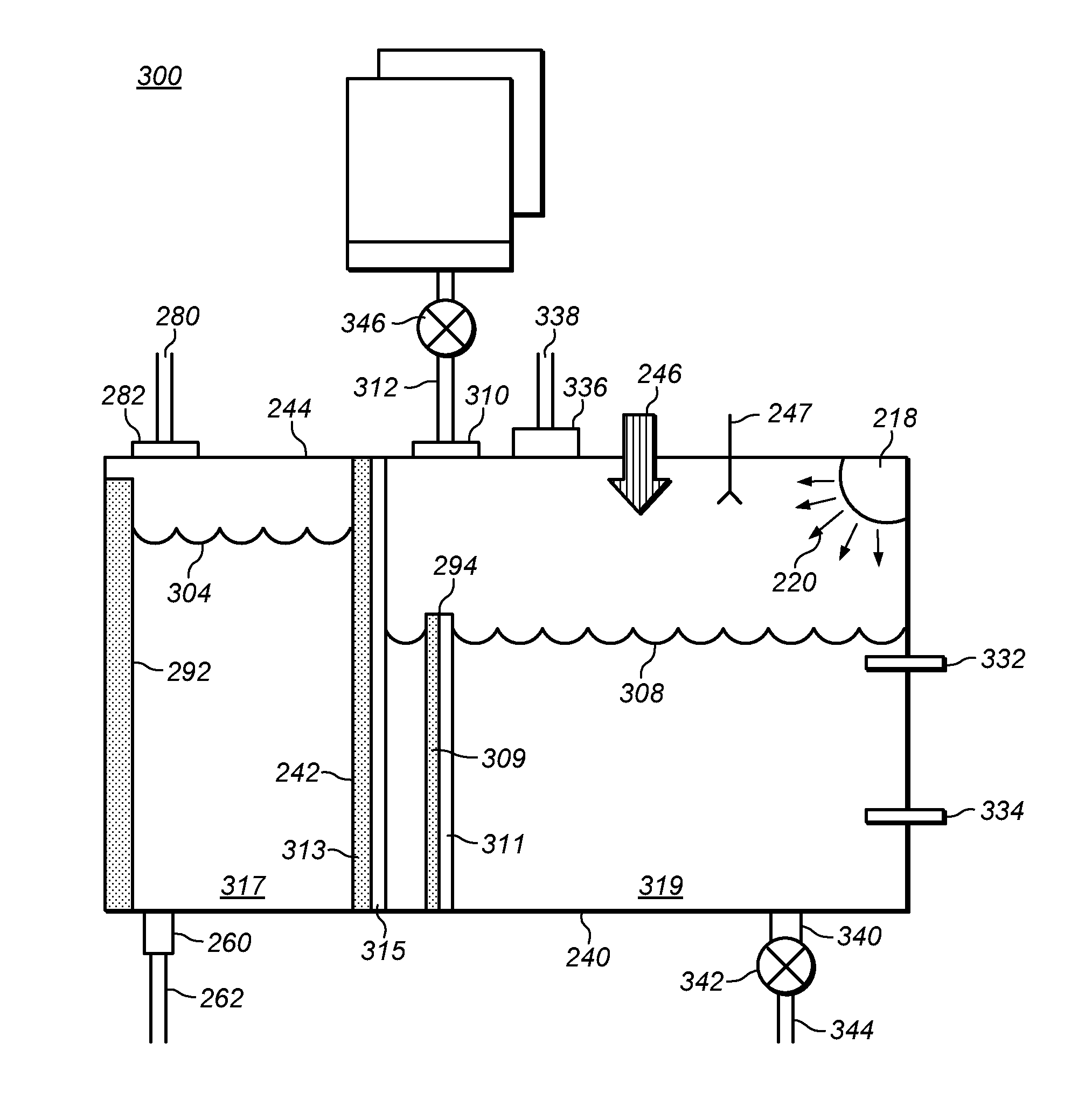
Method of making high purity lithium hydroxide and hydrochloric acid
InactiveUS20110044882A1Simple and economical processEasy to convertElectrolysis componentsEnergy inputElectrolysisIon exchange
The present invention relates to a process for producing high purity lithium hydroxide monohydrate, comprising following steps: concentrating a lithium containing brine; purifying the brine to remove or to reduce the concentrations of ions other than lithium; adjusting the pH of the brine to about 10.5 to 11 to further remove cations other than lithium, if necessary; neutralizing the brine with acid; purifying the brine to reduce the total concentration of calcium and magnesium to less than 150 ppb via ion exchange; electrolyzing the brine to generate a lithium hydroxide solution containing less than 150 ppb total calcium and magnesium, with chlorine and hydrogen gas as byproducts; producing hydrochloric acid via combustion of the chlorine gas with excess hydrogen and subsequent scrubbing of the resultant gas stream with purified water, if elected to do so; and concentrating and crystallizing the lithium hydroxide solution to produce lithium hydroxide monohydrate crystals.
Owner:ROCKWOOD LITHIUM INC
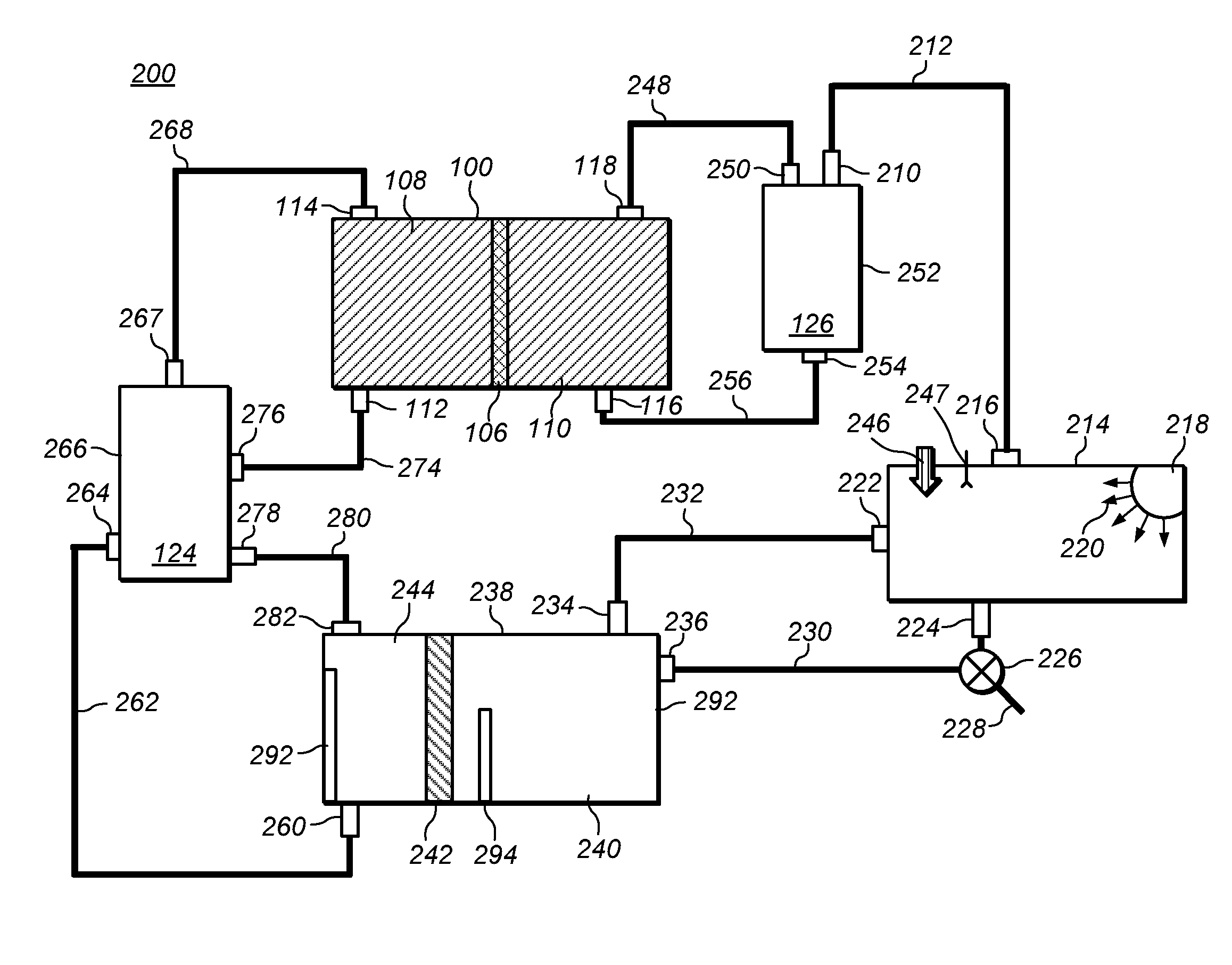
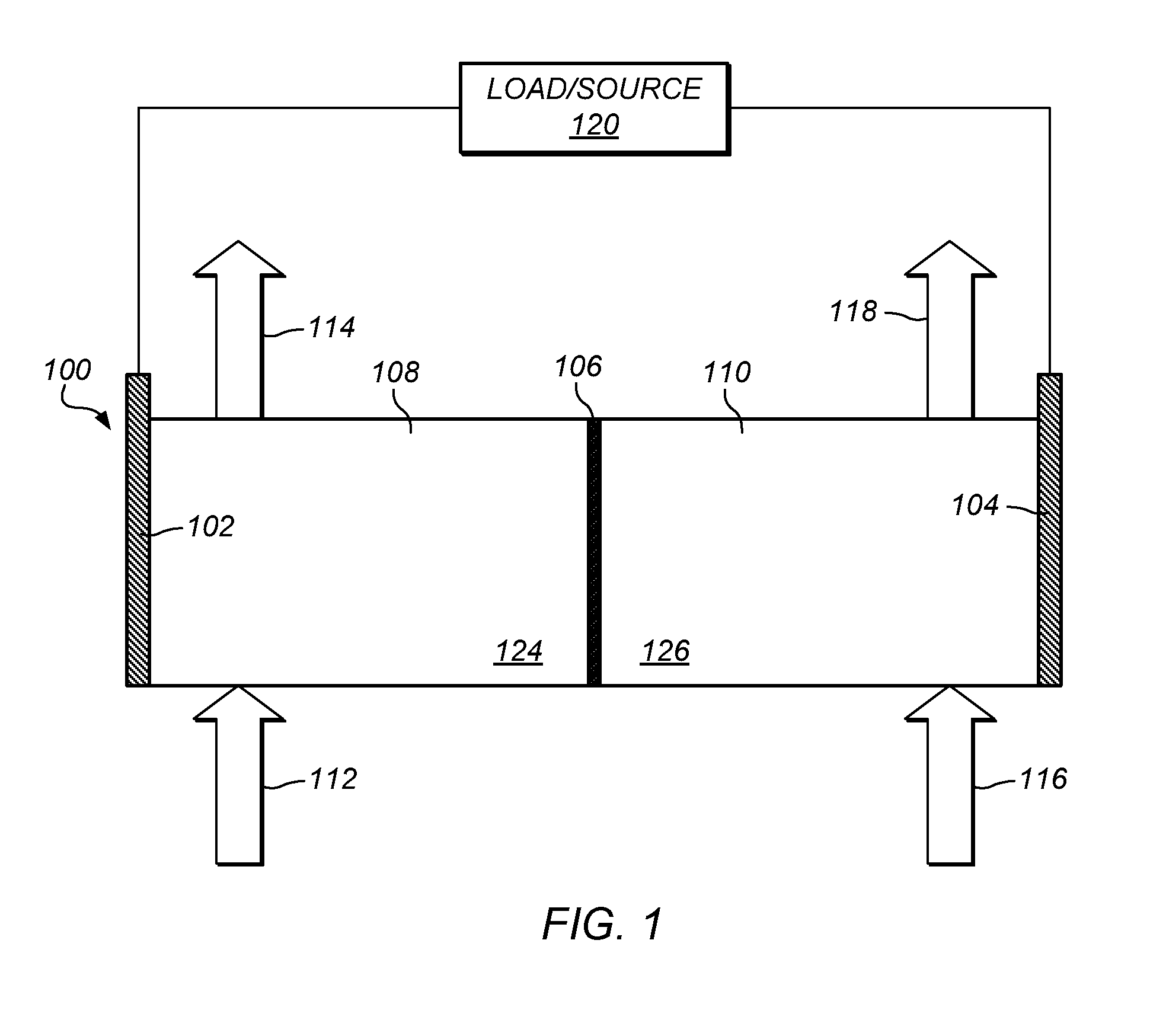
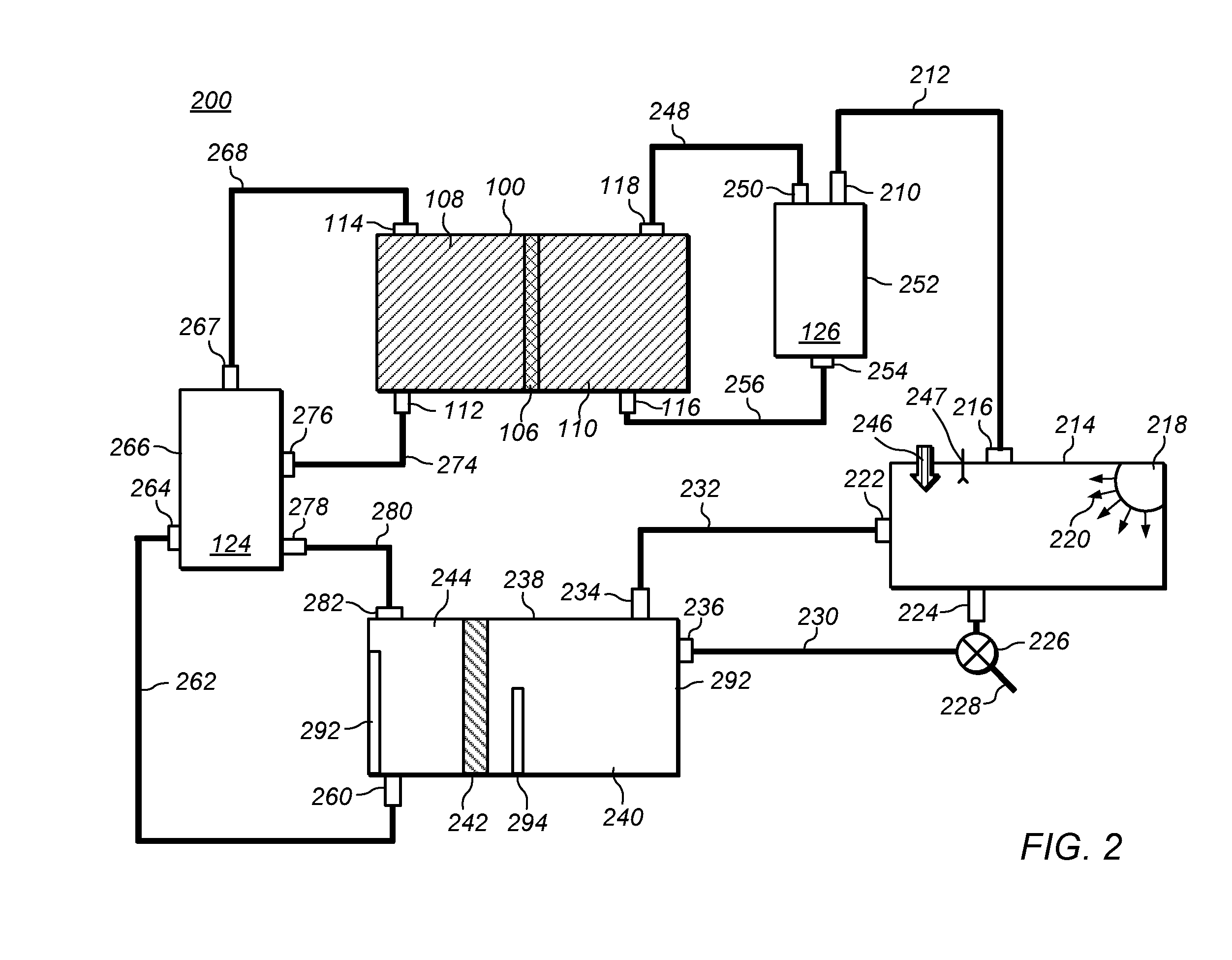
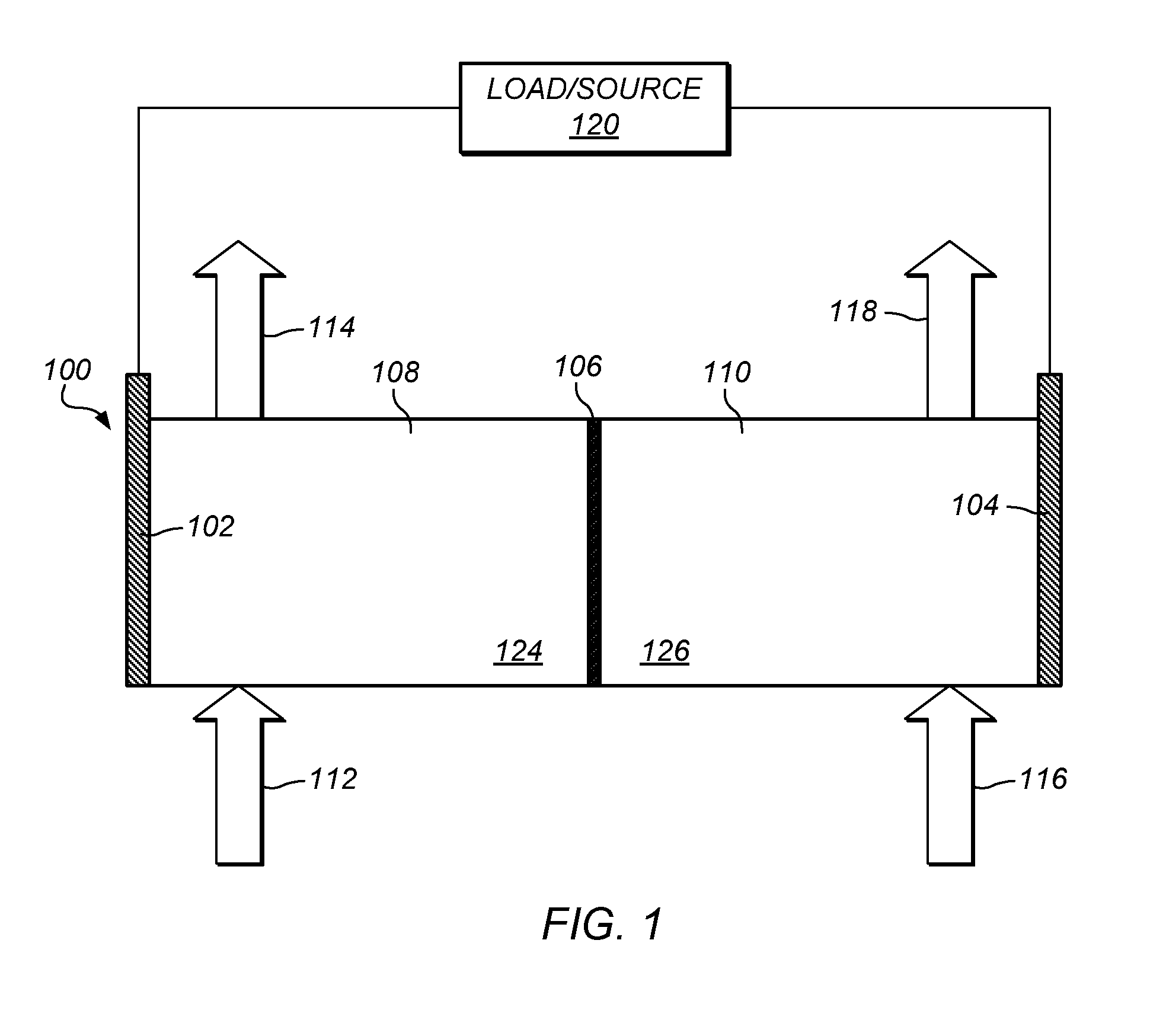
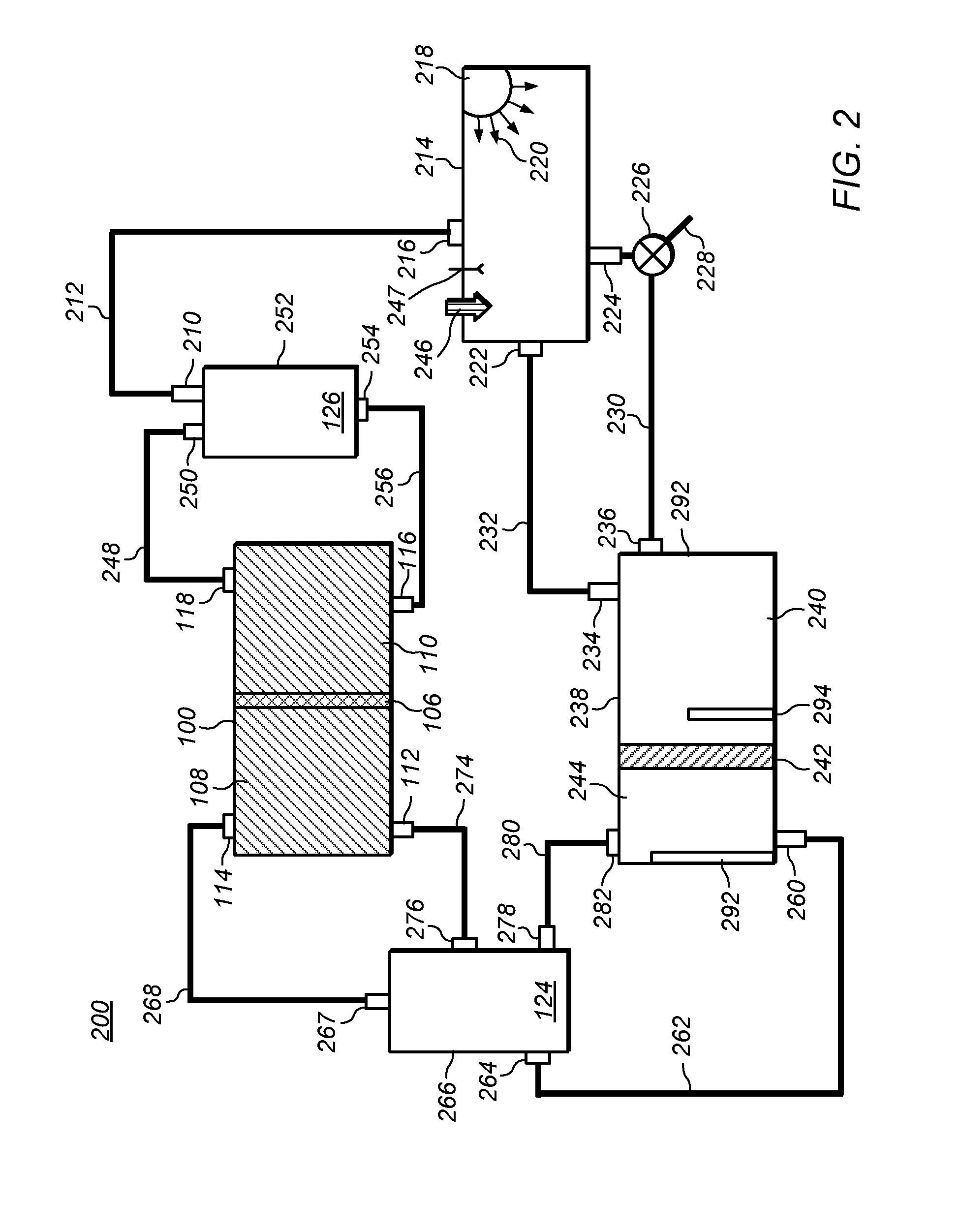
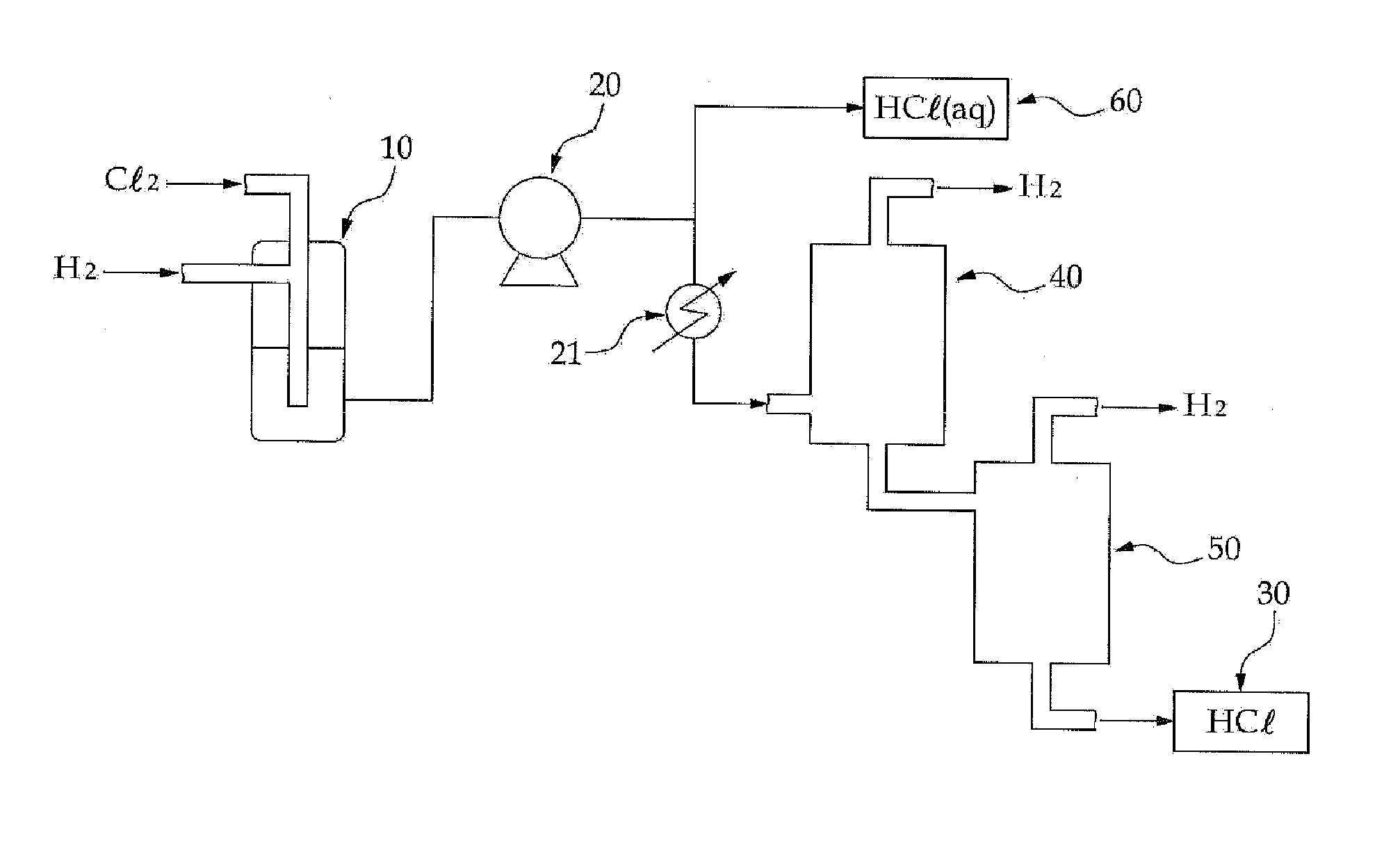
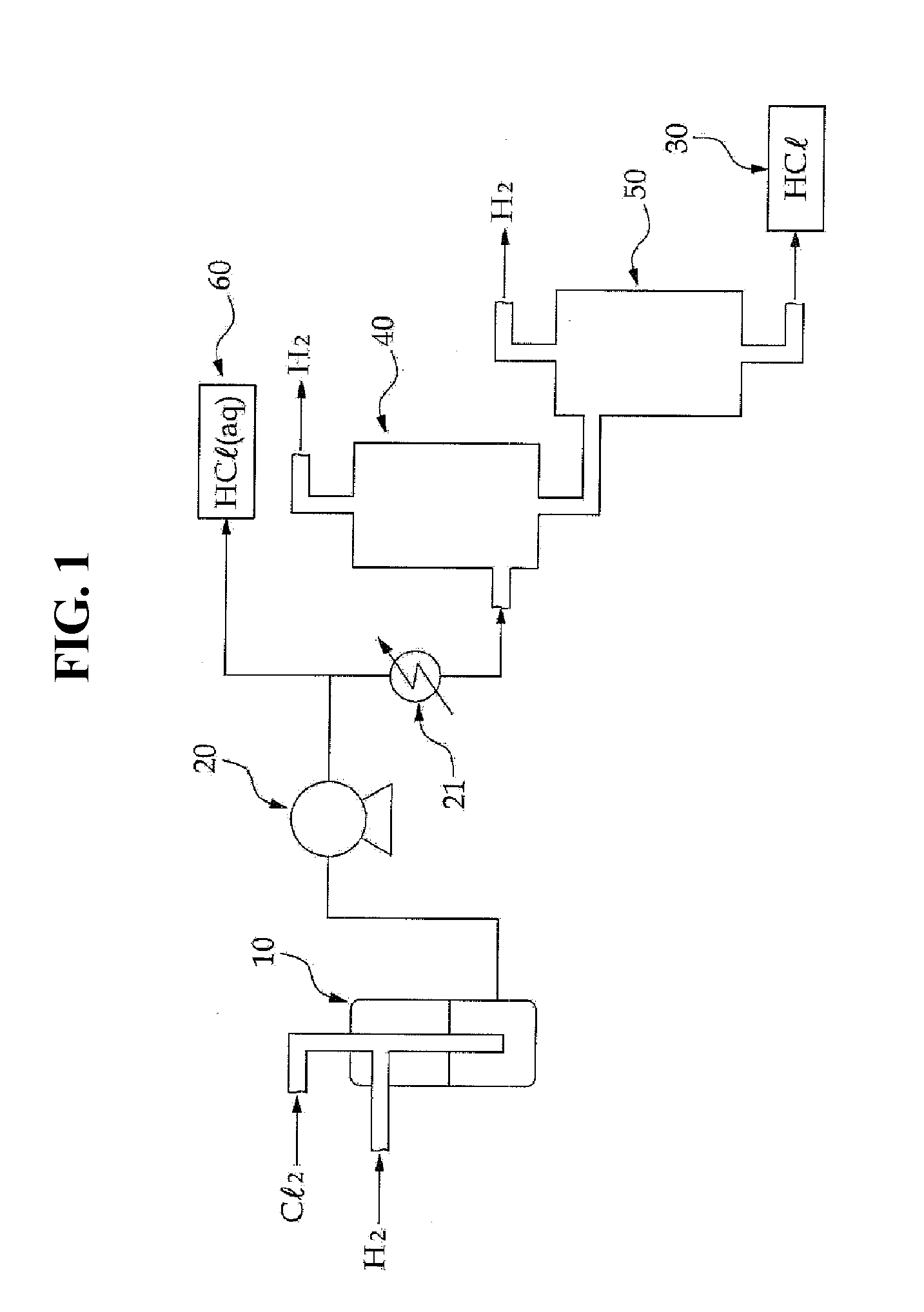
Methods of producing hydrochloric acid from hydrogen gas and chlorine gas
A method of producing HCl from H2 and Cl2 is provided. In some embodiments, the method comprises at least one photochemical chamber placed in fluid communication with at least one source of H2 and at least one source of Cl2. In some embodiments, the photochemical chamber effects the formation of HCl through the use of at least one source of ultraviolet radiation contained therein. In some embodiments, the HCl product may be captured and used as a gas. In some embodiments, the HCl product may be absorbed into water to form an aqueous HCl solution.
Owner:IMERGY POWER SYST
Method of making high purity lithium hydroxide and hydrochloric acid
The present invention relates to a process for producing high purity lithium hydroxide monohydrate, comprising following steps: concentrating a lithium containing brine; purifying the brine to remove or to reduce the concentrations of ions other than lithium; adjusting the pH of the brine to about 10.5 to 11 to further remove cations other than lithium, if necessary; neutralizing the brine with acid; purifying the brine to reduce the total concentration of calcium and magnesium to less than 150 ppb via ion exchange; electrolyzing the brine to generate a lithium hydroxide solution containing less than 150 ppb total calcium and magnesium, with chlorine and hydrogen gas as byproducts; producing hydrochloric acid via combustion of the chlorine gas with excess hydrogen and subsequent scrubbing of the resultant gas stream with purified water, if elected to do so; and concentrating and crystallizing the lithium hydroxide solution to produce lithium hydroxide monohydrate crystals.
Owner:ROCKWOOD LITHIUM INC
Method for a burner and a corresponding device
InactiveUS7666367B1Less spaceMitigate such drawbackChlorine/hydrogen-chloride purificationPhysical/chemical process catalystsCombustorCombustion chamber
The invention relates to a device equipped with a burner for combusting a fuel / oxidant mixture inside a combustion chamber in which a material (3, 3′, 3″, 3′″) is provided that endures a maximum temperature. The inventive device also comprises one or more supply lines (25, 26) for the fuel as well as for the oxidant which are provided for supplying the same into the combustion chamber. The inventive device is characterized in that it is designed for carrying out a combustion with a combustion temperature of the fuel / oxidant mixture that exceeds the maximum temperature. The device is designed in such a way that at least one additional supply line (30) is provided via which an additional gas having, in particular, a low calorific value can be supplied to the combustion chamber. Said additional gas enables the temperature during combustion to be lowered to a value that is less than the maximum temperature.
Owner:SGL CARBON SE
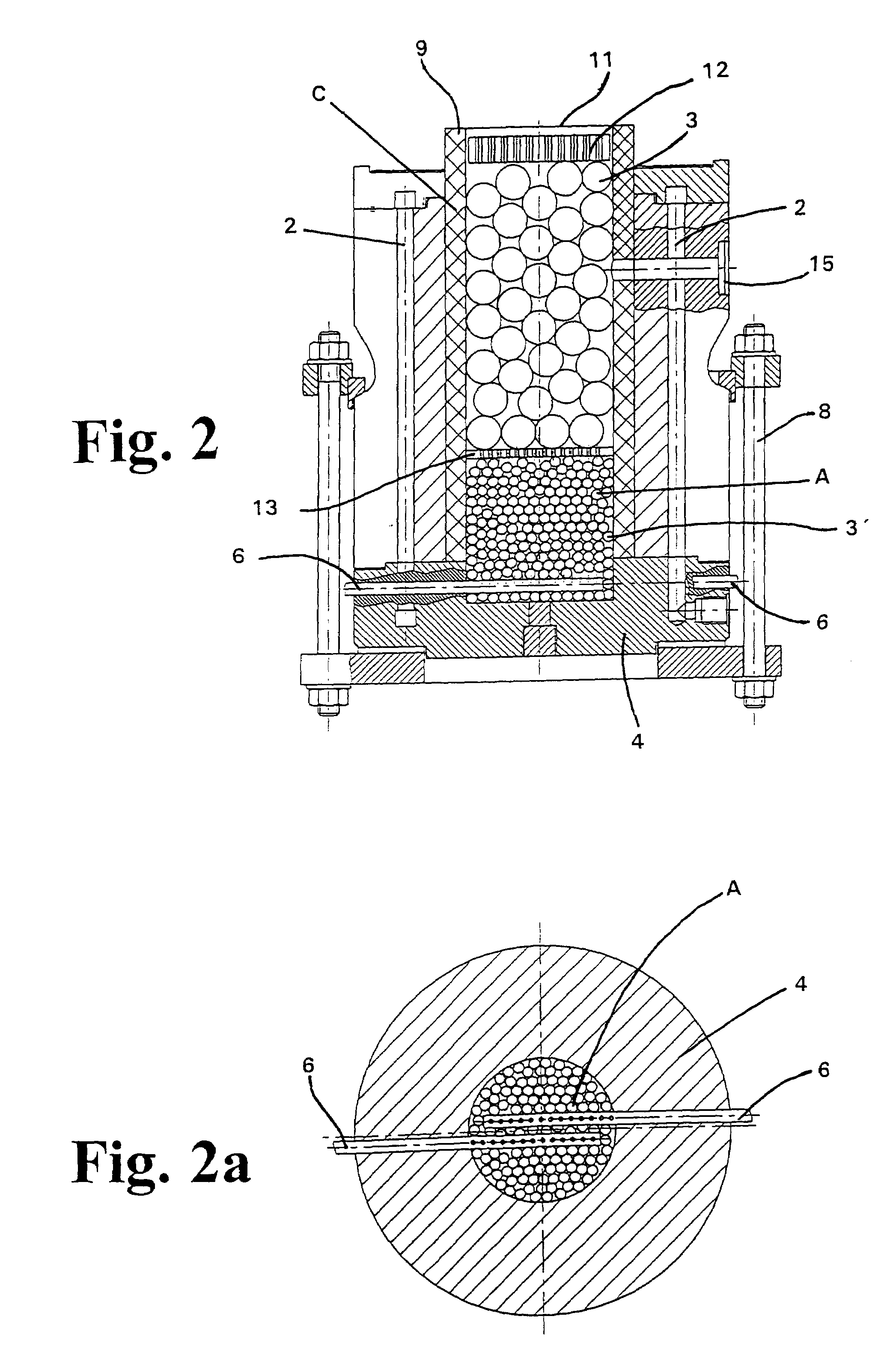
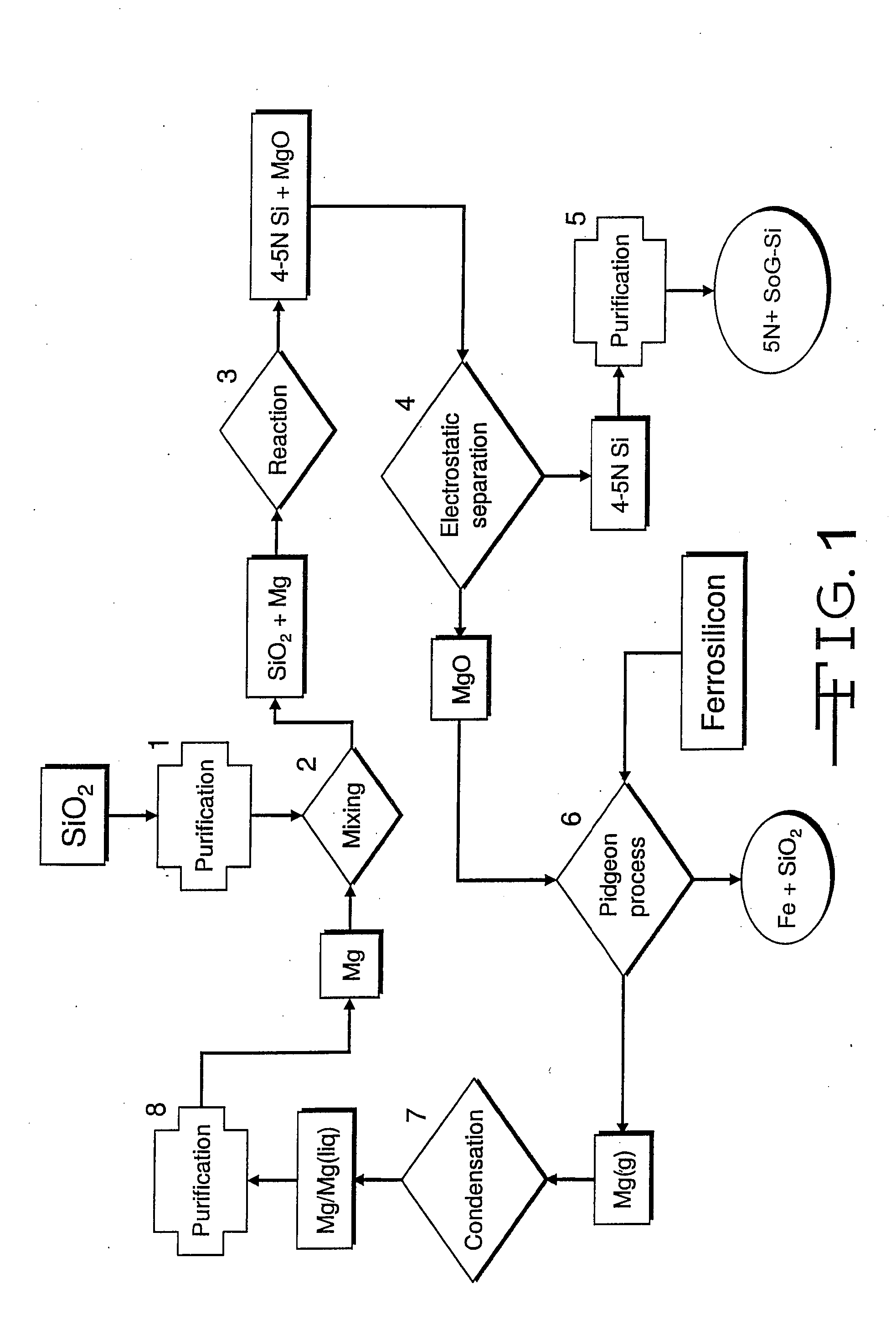
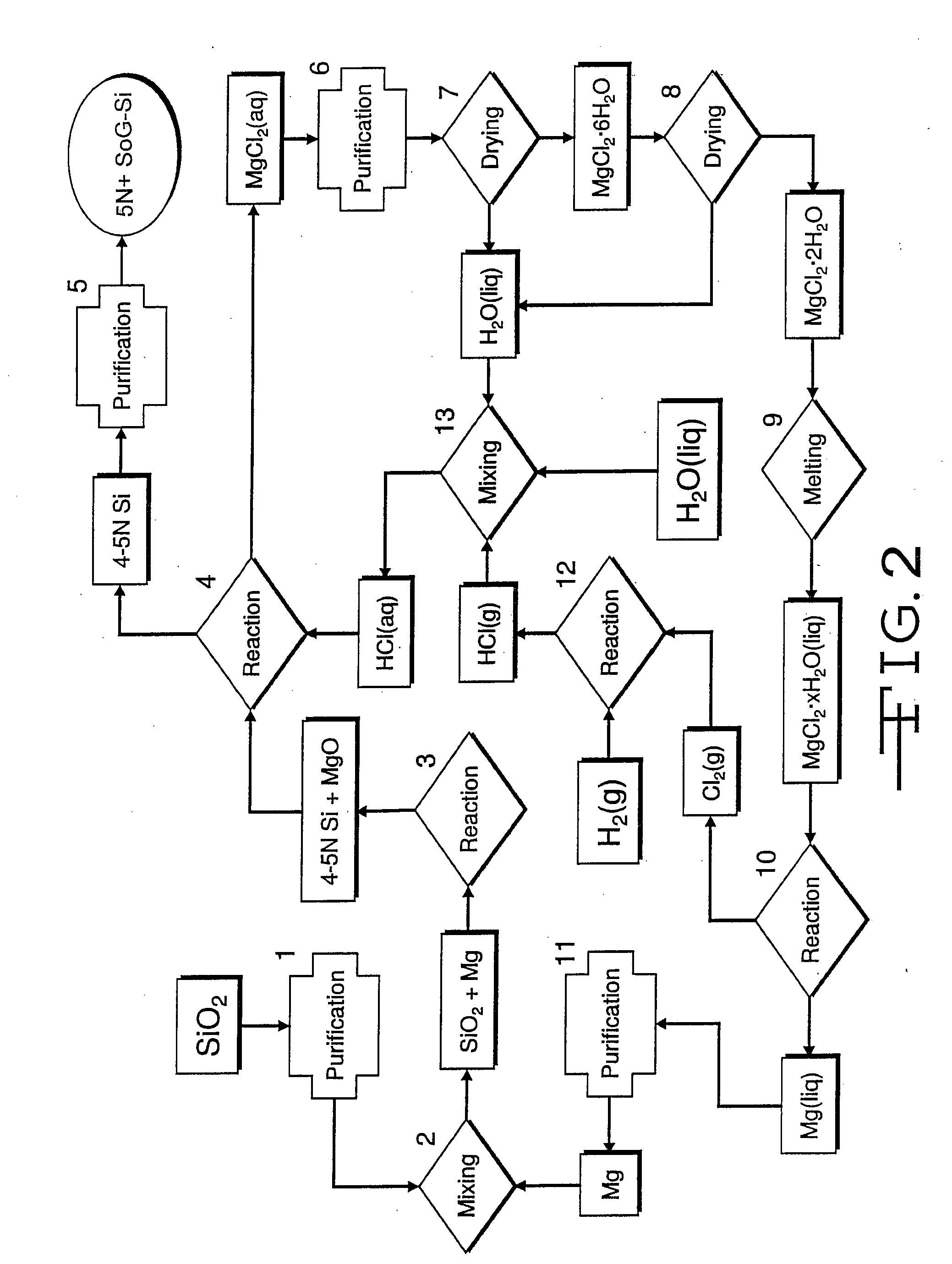
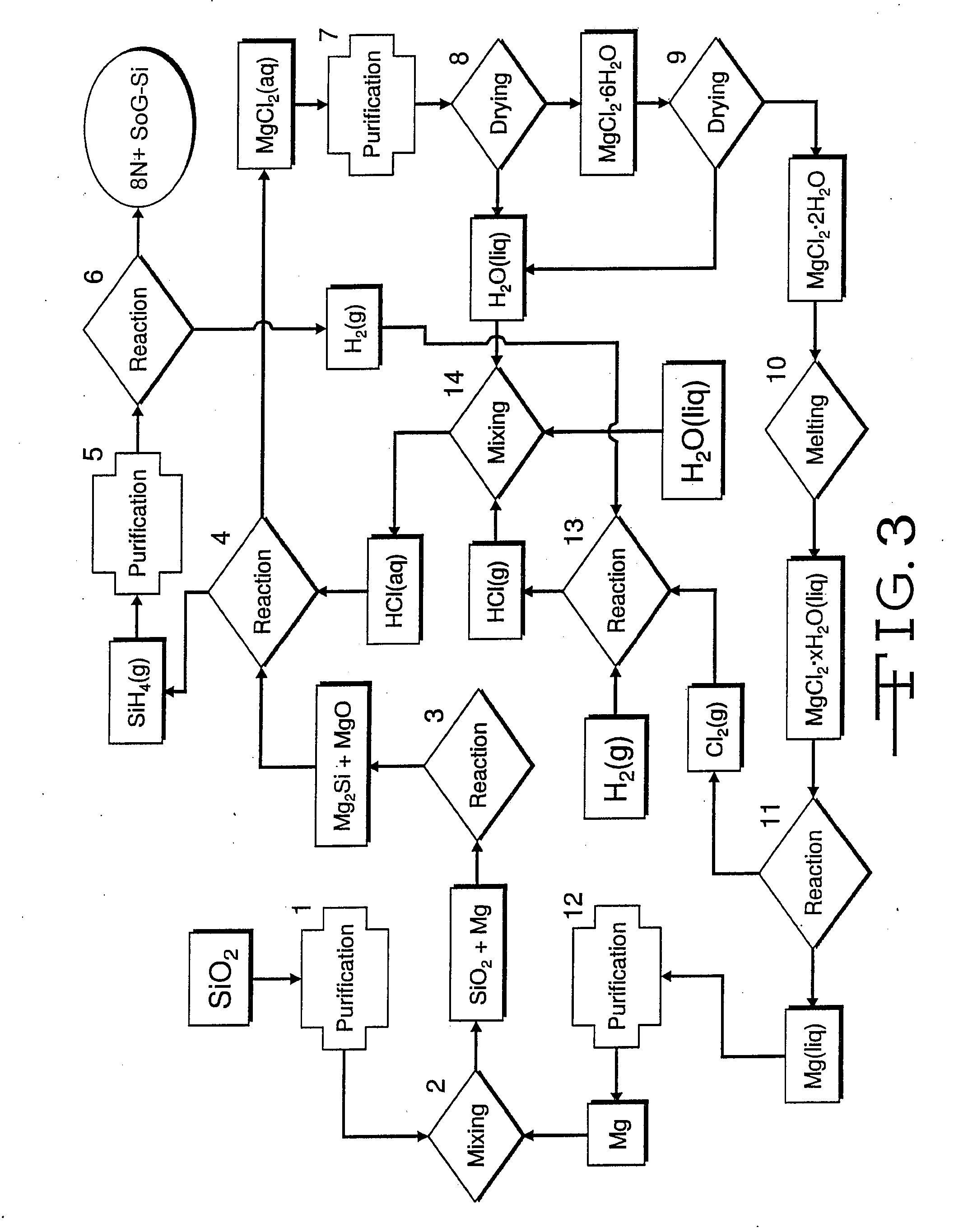
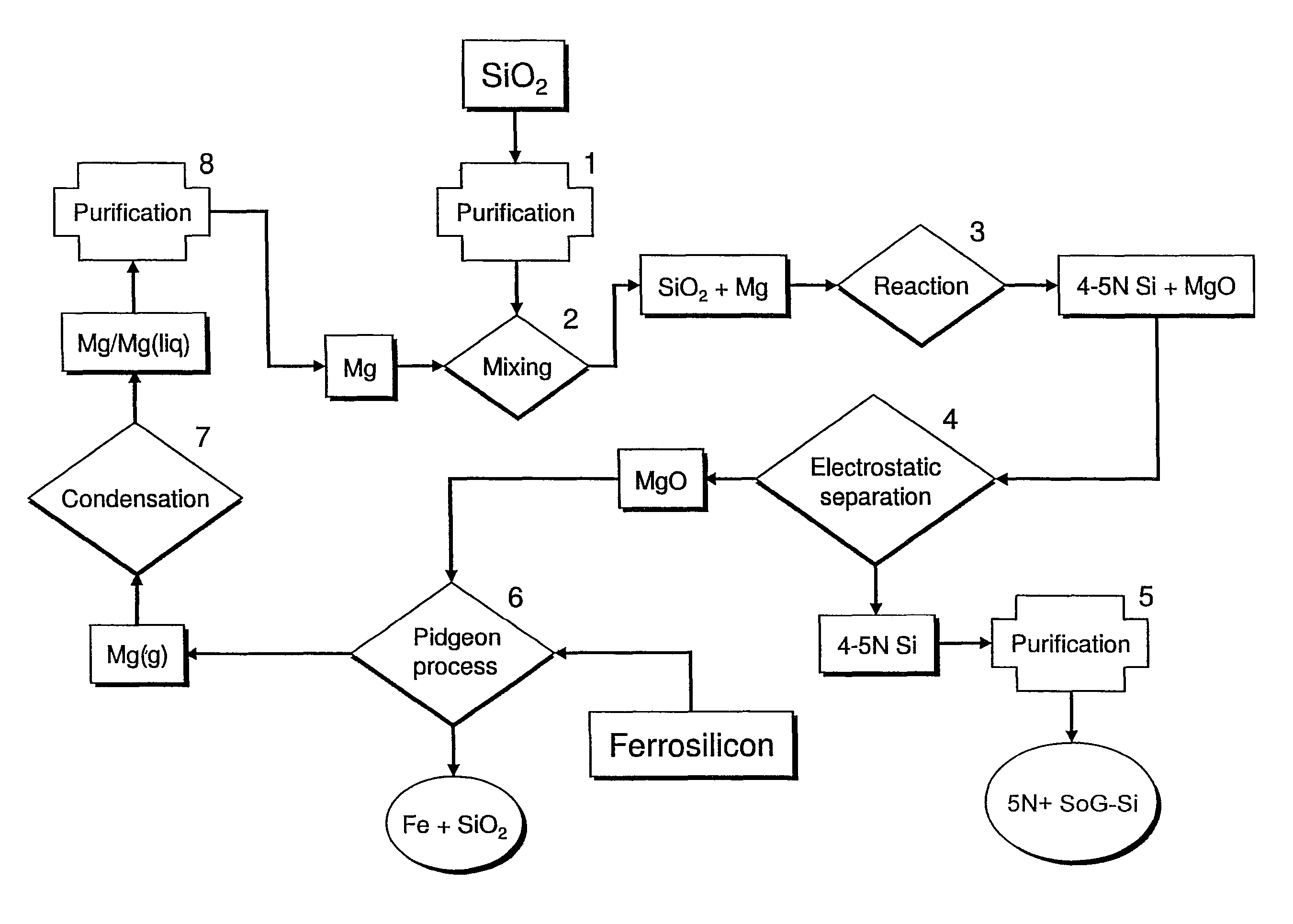
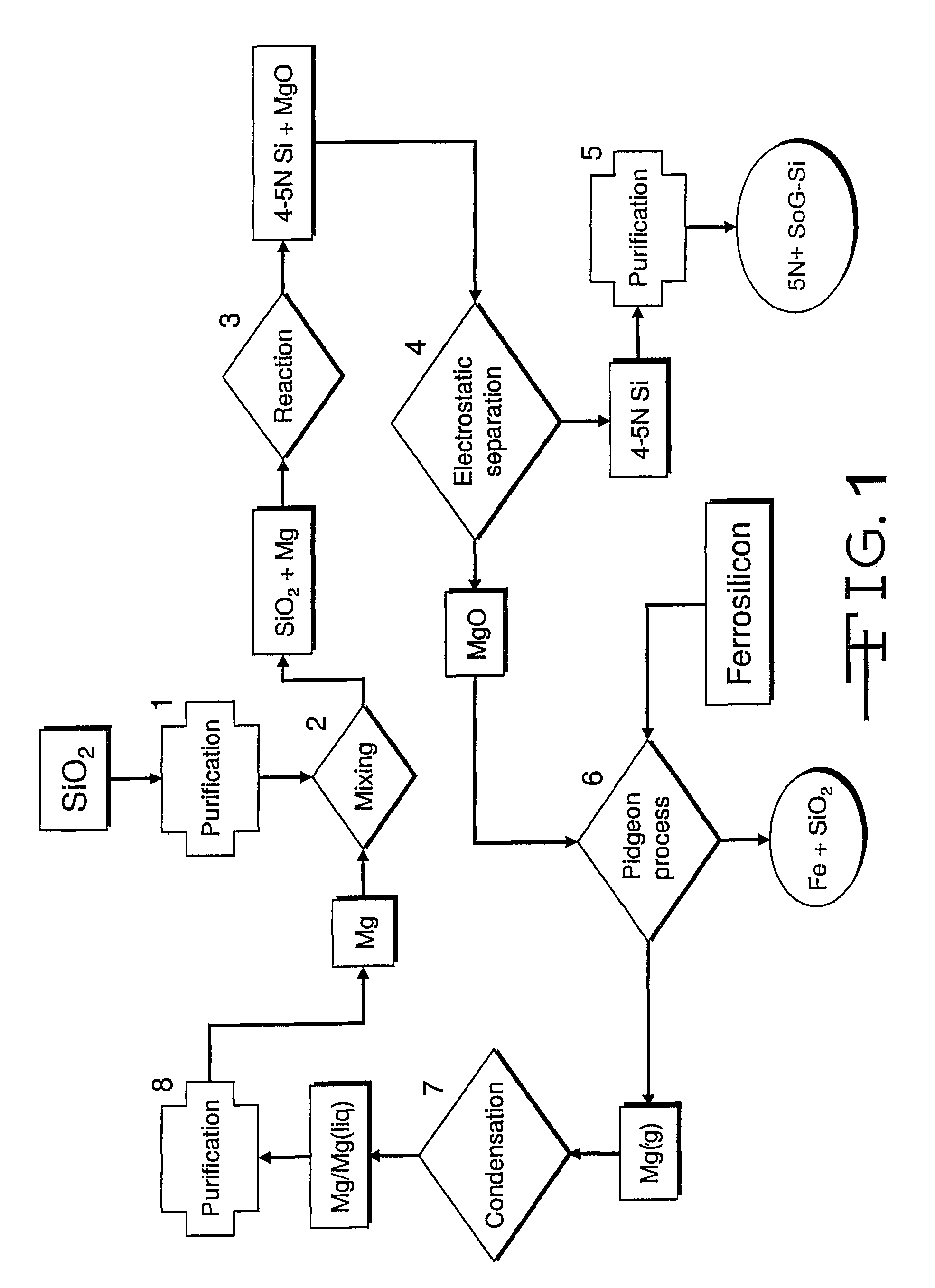
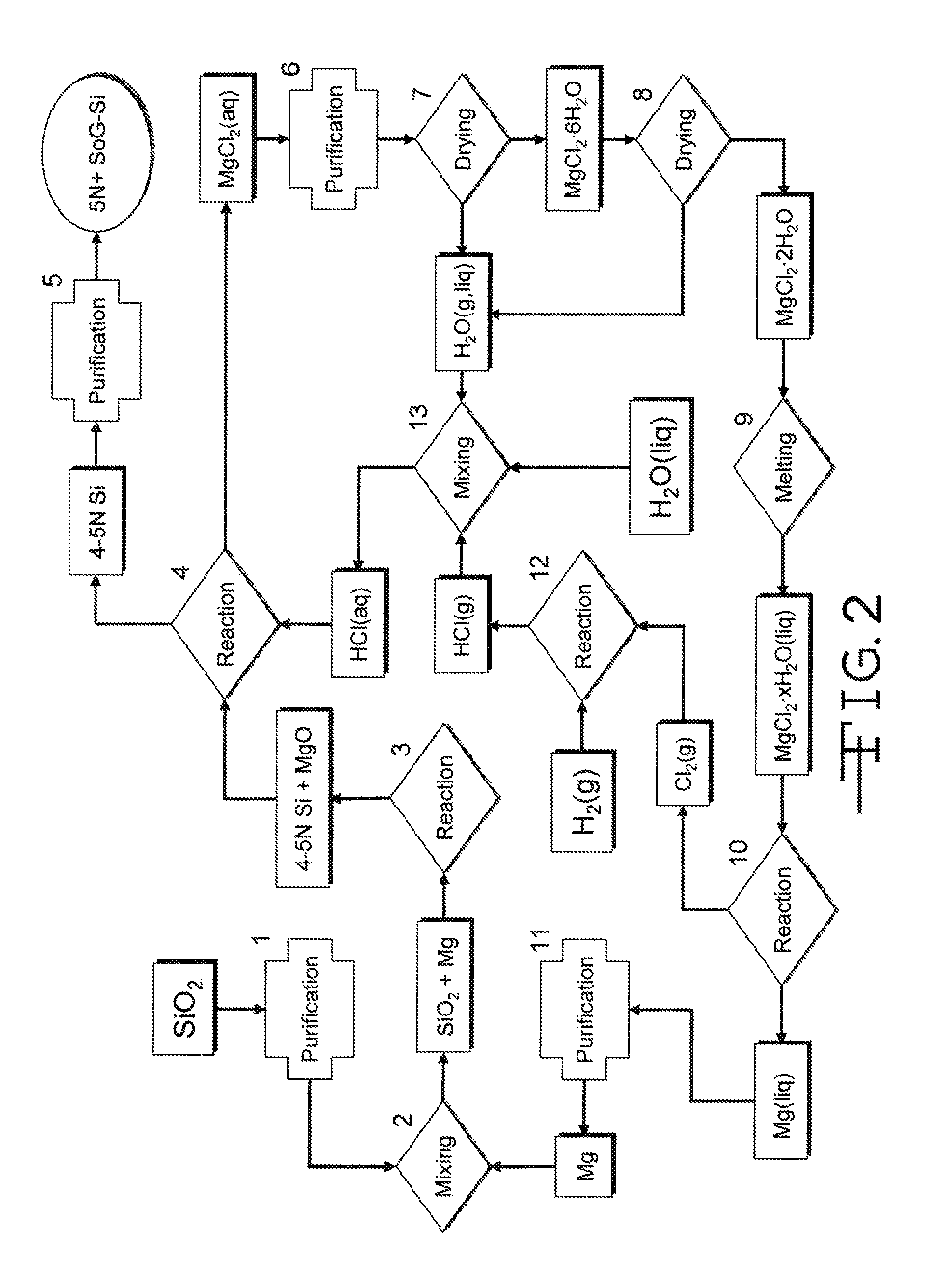
Magnesiothermic Methods Of Producing High-Purity Silicon
Magnesiothermic methods of producing solid silicon are provided. In a first embodiment, solid silica and magnesium gas are reacted at a temperature from 400° C. to 1000° C. to produce solid silicon and solid magnesium oxide, the silicon having a purity from 98.0 to 99.9999%. The silicon is separated from the magnesium oxide using an electrostatic technology. In a second embodiment, the solid silicon is reacted with magnesium gas to produce solid magnesium silicide. The magnesium silicide is contacted with hydrogen chloride gas or hydrochloric acid to produce silane gas. The silane gas is thermally decomposed to produce solid silicon and hydrogen gas, the silicon having a purity of at least 99.9999%. The solid silicon and hydrogen gas are separated into two processing streams. The hydrogen gas is recycled for reaction with chlorine gas to produce hydrogen chloride gas.
Owner:ORION LAB
Methods of producing hydrochloric acid from hydrogen gas and chlorine gas
A method of producing HCl from H2 and Cl2 is provided. In some embodiments, the method comprises at least one photochemical chamber placed in fluid communication with at least one source of H2 and at least one source of Cl2. In some embodiments, the photochemical chamber effects the formation of HCl through the use of at least one source of ultraviolet radiation contained therein. In some embodiments, the HCl product may be captured and used as a gas. In some embodiments, the HCl product may be absorbed into water to form an aqueous HCl solution.
Owner:IMERGY POWER SYST
A comprehensive utilization method for low-grade bauxite
InactiveCN104340998AEfficient separationAchieve cycleElectrolysis componentsAluminium hydroxide preparationFerric hydroxideAluminium hydroxide
The invention relates to a comprehensive utilization method for bauxite and particularly relates to a comprehensive utilization method for low-grade bauxite. The method includes following steps of: (1) mixing the low-grade bauxite with hydrochloric acid after ore grinding; (2) performing solid liquid separation and washing after the reaction is cooled; (3) adding the solution obtained in the step (2) into a sodium hydroxide solution to obtain an aluminium hydroxide precipitate, an iron hydroxide precipitate and a sodium chloride solution, performing solid liquid separation, and washing; (4) preparing the aluminium hydroxide and iron hydroxide solid obtained in the step (3) into metallurgy-level aluminum oxide and high-iron slag through a simple Bayer process; (5) subjecting the sodium chloride solution obtained in the step (3) to electrolysis by an ionic exchange membrane electrolytic cell to obtain hydrogen, chlorine and a sodium hydroxide solution; and (6) returning the sodium hydroxide solution that is discharged from an ionic membrane cathode zone in the step (5) into the step (3) and recycling. The method is obvious in environment protection effects, and effectively separates aluminum, iron and silicon in the low-grade bauxite to achieve comprehensive utilization.
Owner:SHENYANG ALUMINIUM MAGNESIUM INSTITUTE
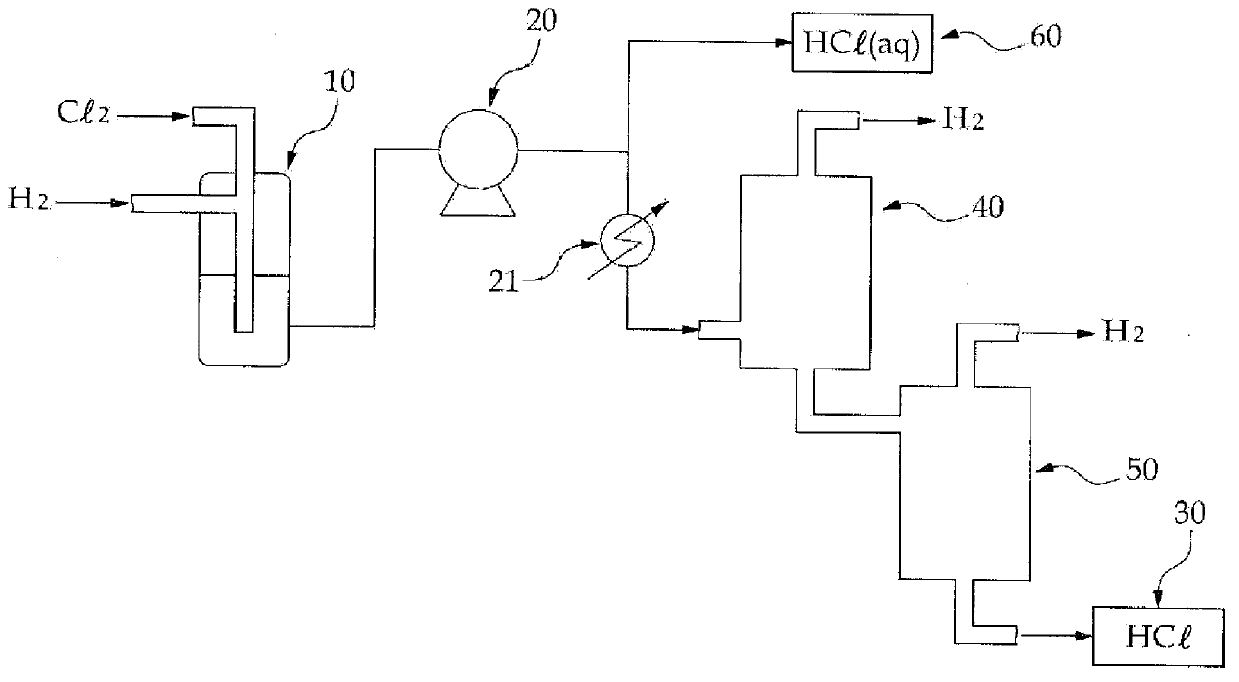
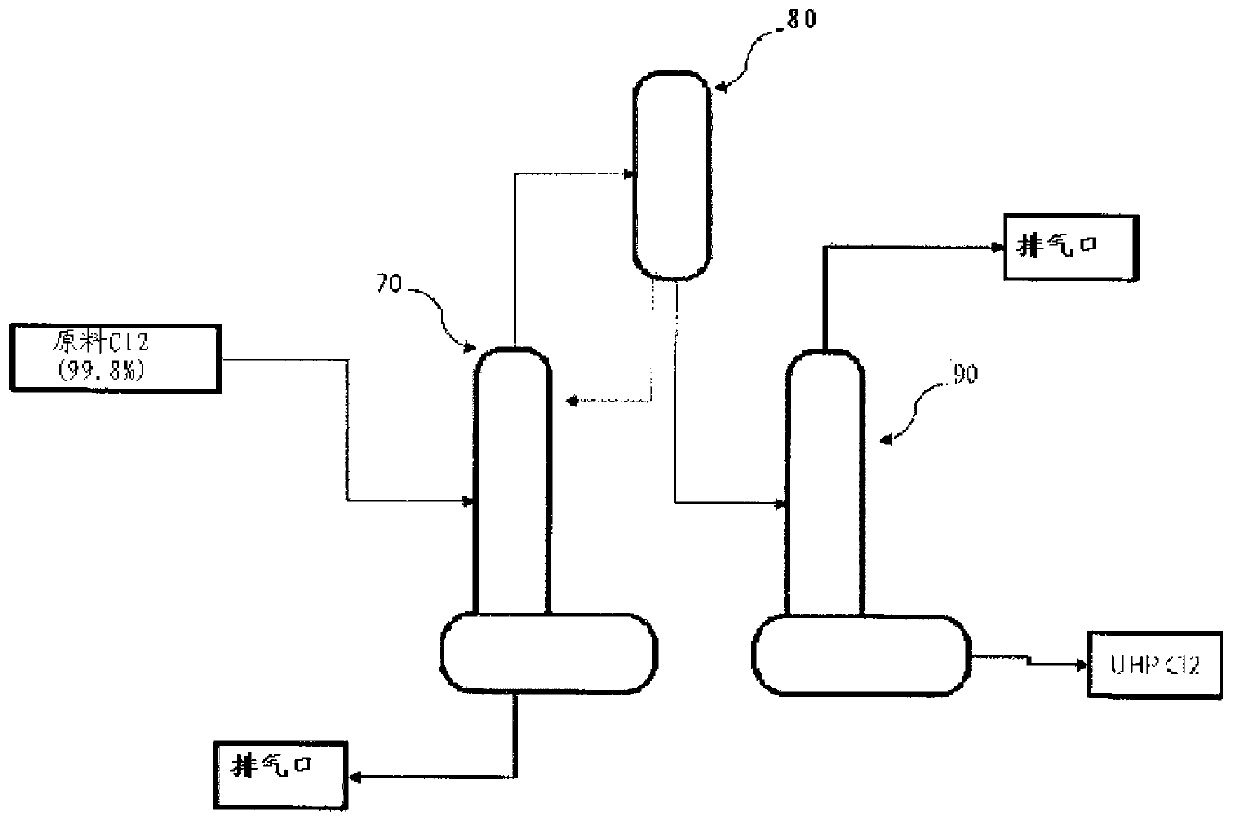
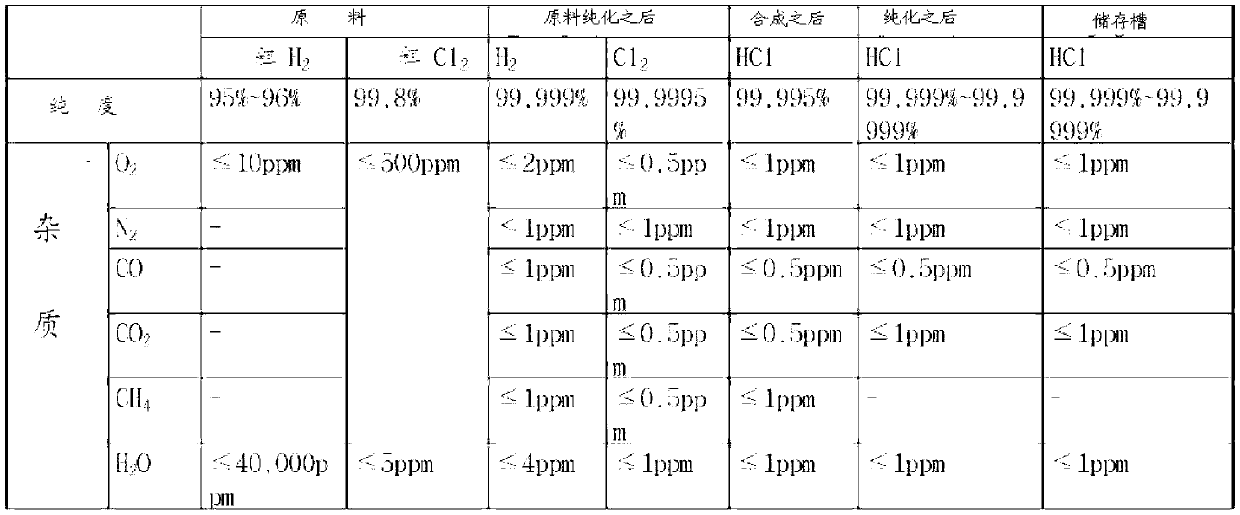
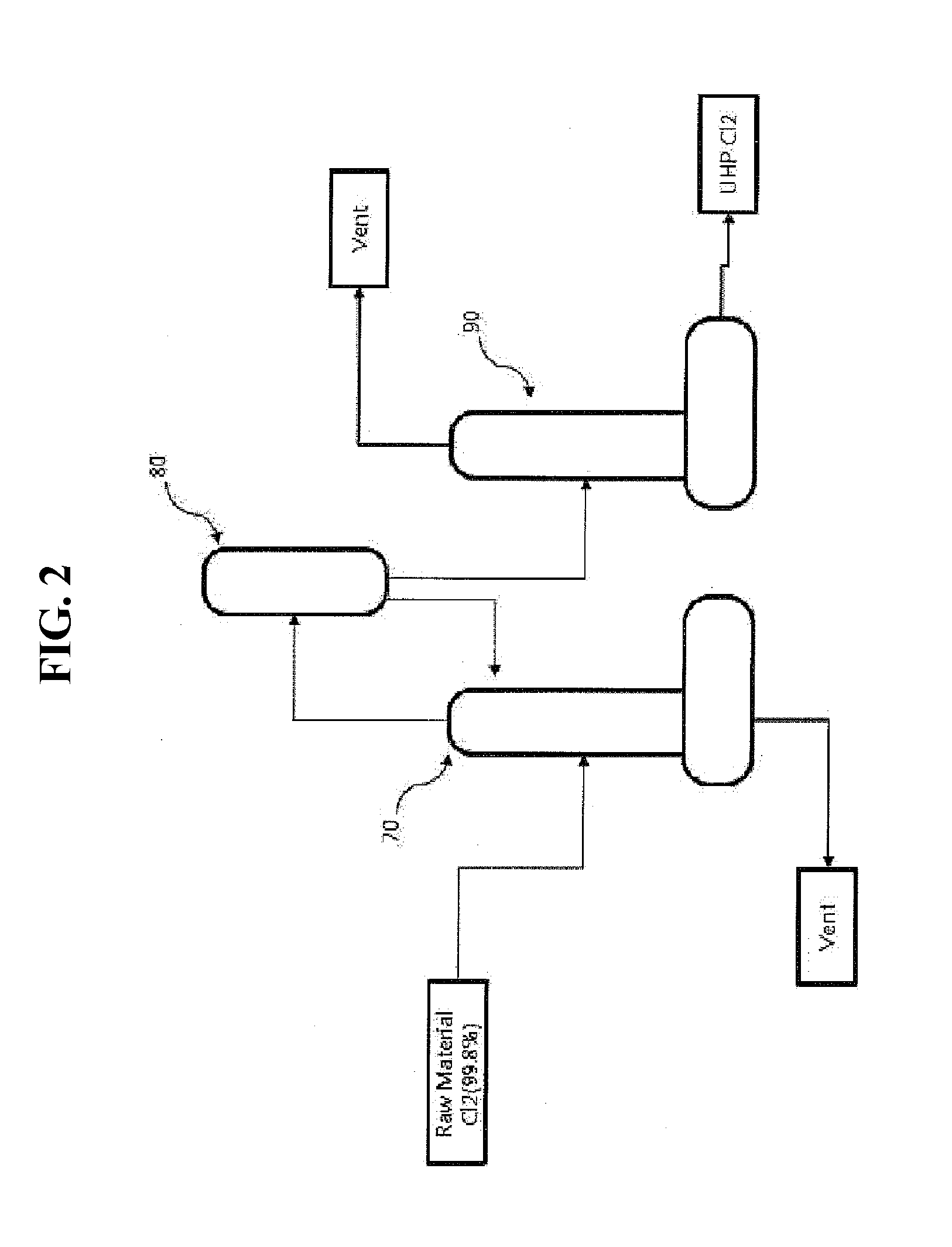
Method and system for preparing high-purity hydrogen chloride
InactiveCN103221336AReduce consumptionConducive to simplificationIon-exchange process apparatusOrganic compounds purification/separation/stabilisationHydrogenDecreased energy
The present invention provides a method and system for preparing high-purity hydrogen chloride, the method comprising the steps of: purifying the raw materials, crude hydrogen and crude chlorine, to a purity of at least 99.999%; synthesizing hydrogen chloride by reacting the purified hydrogen and chlorine at a temperature in the range of from 1,200 to 1,400 DEG C, the hydrogen being introduced in excess by a molar ratio relative to the chlorine; compressing the hydrogen chloride so as to convert same to a liquid; and refining the hydrogen chloride and isolating the surplus hydrogen through fractional distillation. The method and system for preparing high-purity hydrogen chloride of the present invention can readily produce hydrogen chloride of from 3N (99.9%) to 6N (99.9999%) in large quantities and at relatively low cost depending on the raw materials and the degree of purity of the product, and can provide an environmentally friendly preparation process that dramatically decreases energy consumption.
Owner:HONG IN CHEM +1
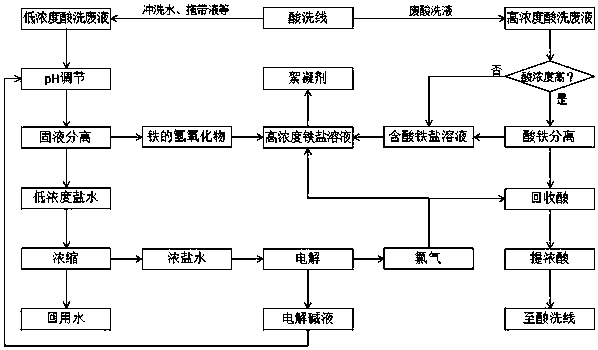
Green recycling method for steel pickling waste liquor
ActiveCN108455680ASolve the problem that the iron content is low and it is not suitable for the preparation of flocculantsIncrease profitElectrolysis componentsIron oxides/hydroxidesHigh concentrationSaline water
The invention discloses a green recycling method for steel pickling waste liquor. The green recycling method is characterized by specially comprising the following steps: neutralizing low-concentration pickling waste liquor with inorganic base, thereby obtaining ferric hydroxide or ferrous hydroxide precipitates and low-concentration saline water; performing acid and iron separation on high-concentration pickling waste liquor, thereby obtaining recycled acid and a ferric acid containing solution; dehydrating ferric hydroxide or ferrous hydroxide precipitates, and then, adding the ferric acid containing solution to obtain high-concentration iron salt solution; concentrating the low-concentration saline water to obtain concentrated saline water and recyclable water; electrolyzing concentrated saline water to prepare chlorine gas and an inorganic base solution; circulating the inorganic base solution for neutralizing pickling waste liquor; leading chlorine gas into the recycling acid to improve concentration of hydrochloric acid, and recycling concentrated recycled acid to a pickling line; and leading chlorine gas into a high-concentration iron salt solution to prepare a flocculatingagent. The green recycling method has the advantages that: a utilization rate of the pickling waste liquor is expected to be greatly increased, emission of three wastes of gas, liquid and solid is avoided throughout the process, and the method is economical, green and environmentally friendly.
Owner:江苏宝钢精密钢丝有限公司 +1
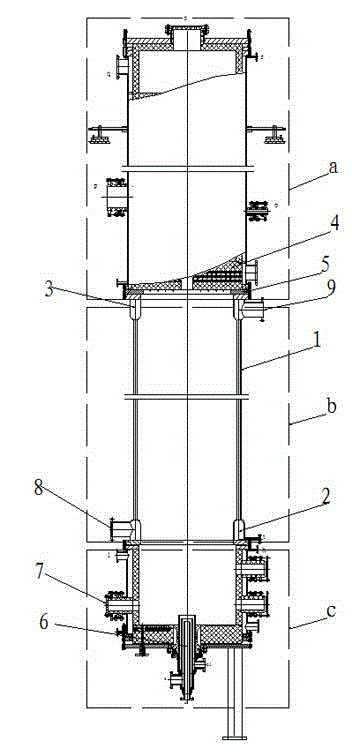
Novel combined type hydrogen chloride synthetic furnace with byproduct of high-pressure steam
ActiveCN104445071AExtended service lifeSolve pressureHydrogen chloride preparationHydrogen chlorideEngineering
The invention relates to a novel combined type hydrogen chloride synthetic furnace with a byproduct of high-pressure steam. The novel combined type hydrogen chloride synthetic furnace comprises a synthetic furnace and a cooler at the top of the synthetic furnace, wherein the synthetic furnace is divided into an upper section, a middle section and a lower section; each of the upper section and the lower section consists of a graphite cylinder and a steel shell sleeved outside the cylinder; and the middle section is the steel shell which is formed by a plurality of vertical steel condensation tubes in annular sealed matching. The novel combined type hydrogen chloride synthetic furnace provided by the invention has the following advantages that the middle section of the synthetic furnace is changed from an original structure comprising a steel shell and an inner graphite cylinder into a steel shell structure which is formed by the plurality of vertical steel condensation tubes in annular sealed matching, so that the pressure for generating steam is improved; and meanwhile, a flow guide structure is additionally arranged at a flange connection part between the upper section and the middle section, and a flow guide ring is arranged at the lower end surface of a graphite heat exchange block, so that hydrochloric acid is prevented from corroding the steel shell by spreading to a flange surface of a flange and then being in contact with the steel shell of the lower section of the cylinder, and the service life of the synthetic furnace is improved.
Owner:NANTONG STAR GRAPHITE EQUIP CO LTD
Generation of hydrogen on demand
InactiveUS20110094894A1Smoothes variabilitySimple and safe processSamplingRegenerative fuel cellsElectrolysisSolar power
The methods and systems for producing hydrogen on demand use aluminum, a heat source and an electrical source, such as, but not limited to, solar power. The heat source and electrical source is used to produce chemical intermediates from sodium chloride via electrolysis. The chemical intermediates from the sodium chloride may be reacted with aluminum to produce hydrogen. The on-demand hydrogen systems can generate a continuous stream of hydrogen that can power a home or business. Alternatively, or in addition, the on-demand hydrogen system can be incorporated into a vehicle to power the vehicle.
Owner:MASON DENNIS B
Method and device for preparing hydrochloric acid and purifying quartz sand
ActiveCN106219488AQuality assuranceHigh purityChlorine/hydrogen-chloride purificationSilicaMetal chlorideHydrogen
The invention provides a method and a device for preparing hydrochloric acid and purifying quartz sand. The method comprises the following steps: igniting hydrogen and chlorine in a synthetic furnace, carrying out a reaction to produce hydrogen chloride gas, releasing heat, and controlling the temperature of hydrogen chloride gas at the outlet of the synthetic furnace to be 500-1300 DEG C; heating cooling air in a gas cooling device, introducing the air into the bottom of a drying device filled with semi-finished quartz sand, allowing hot air to flow from the bottom upwards, drying the semi-finished quartz sand, and taking the moisture therein; allowing the dried semi-finished quartz sand into a chlorination impurity removal device by the drying device, and completing purification of the quartz sand; allowing the hydrogen chloride gas and metal chloride gas in the chlorination impurity removal device to flow out of the top, and allowing the gas to enter the gas cooling device. According to the method disclosed by the invention, the whole quartz sand purification process can be energy-saving and low in cost on the premise of guaranteeing the quality of synthetic hydrochloric acid, and the purified quartz sand is high in purity.
Owner:田晋丞
Means and method of chemical production
InactiveUS20050072687A1Provide flexibilityImprove balanceElectrolysis componentsHydrogen chloride preparationPotassium hydroxideBleach
Disclosed is a process for manufacturing bleach (or sodium hypochlorite) and caustic potash (or KOH) without the need for shipping or storing chlorine gas. Specifically, the present invention relates to the manufacture of potassium hydroxide and chlorine gas, through several process options, for the manufacture of sodium hypochlorite (or bleach), hydrochloric acid (HCl) and / or other chlorinated compounds. The disclosed process allows operating flexibility based on chlorine demand, reduces capital costs and eliminates the need for the transportation and storage of chlorine gas.
Owner:HUBBARD FR G
Method for preparing high-purity hydrogen chloride
ActiveCN106276796AImprove adsorption capacityImprove the ability to absorb chlorine gasChlorine/hydrogen-chloride purificationHydrogen chloride preparationHydrogenSorbent
The invention relates to a method for preparing high-purity hydrogen chloride. Firstly, an efficient adsorbent is prepared, electronic-grade hydrogen and electronic-grade chlorine enter a synthesis furnace to synthesize hydrogen chloride, the synthesized hydrogen chloride enters an adsorption tower containing the efficient adsorbent for refining and dechlorination, the adsorbed hydrogen chloride gas is cooled and liquefied, hydrogen and noncondensable gas are removed, and a high-purity hydrogen chloride product is obtained.
Owner:ZHEJIANG BRITECH CO LTD
Magnesiothermic methods of producing high-purity silicon
Magnesiothermic methods of producing solid silicon are provided. In a first embodiment, solid silica and magnesium gas are reacted at a temperature from 400° C. to 1000° C. to produce solid silicon and solid magnesium oxide, the silicon having a purity from 98.0 to 99.9999%. The silicon is separated from the magnesium oxide using an electrostatic technology. In a second embodiment, the solid silicon is reacted with magnesium gas to produce solid magnesium silicide. The magnesium silicide is contacted with hydrogen chloride gas or hydrochloric acid to produce silane gas. The silane gas is thermally decomposed to produce solid silicon and hydrogen gas, the silicon having a purity of at least 99.9999%. The solid silicon and hydrogen gas are separated into two processing streams. The hydrogen gas is recycled for reaction with chlorine gas to produce hydrogen chloride gas.
Owner:ORION LAB
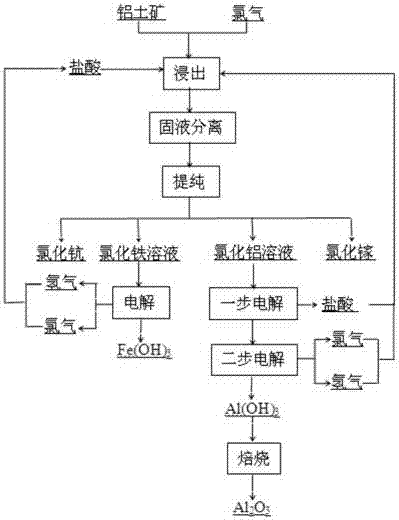
Method for preparing aluminum oxide through hydrochloric acid leaching and two-stage electrolysis of bauxite and comprehensively utilizing aluminum oxide
ActiveCN107128959AHigh degree of automationHigh purityAluminium compoundsRare earth metal chloridesAluminium chlorideElectrolysis
The invention relates to a method for preparing aluminum oxide through hydrochloric acid leaching and two-stage electrolysis of bauxite and comprehensively utilizing aluminum oxide. The method comprises the following steps: carrying out hydrochloric acid leaching, solid-liquid separation and purification on bauxite on bauxite, so as to obtain an iron chloride mixed solution and an aluminum chloride mixed solution; respectively separating and purifying the iron chloride mixed solution and the aluminum chloride mixed solution, so as to obtain scandium chloride, gallium chloride, an aluminum chloride water solution and an iron chloride water solution; setting electrolytic voltage and current density, and respectively carrying out two-stage electrolysis on the aluminum chloride water solution and the iron chloride water solution, so as to respectively obtain aluminum hydroxide, hydrogen and chlorine as well as iron hydroxide, hydrogen and chlorine; preparing a hydrochloric acid solution by using the generated hydrogen and chlorine, and returning the hydrochloric acid solution to a leaching stage for recycling; and roasting aluminum hydroxide, so as to obtain metallurgy level aluminum oxide or chemical aluminum oxide. Compared with a traditional acid method, the electrolysis method for recycling aluminum oxide in the bauxite and processing the bauxite has the advantages that the evaporation step and equipment, the concentration step and equipment are omitted, the operation is simplified, meanwhile, the cost is substantially lowered, and the product has relatively high purity.
Owner:NORTHEASTERN UNIV
Green and environment-friendly white carbon black and caustic soda circulation combined production method
ActiveCN109678164AReduce manufacturing costHigh economic valueSilicaElectrolysis componentsEvaporation PurificationElectrolysis
The invention discloses a green and environment-friendly white carbon black and caustic soda circulation combined production method. The method comprises the following steps: (1), performing a reaction on sodium silicate and hydrochloric acid to obtain a silica precipitation filter cake and a sodium chloride solution; (2), washing and slurrying the silica precipitation filter cake, and performingdrying, crushing and packaging to obtain a finished white carbon black product; (3), treating the sodium chloride solution by an RO membrane twice to obtain a sodium chloride solution with concentration of 12%; (4), performing multi-effect evaporation purification on the sodium chloride solution with concentration of 12% to obtain a sodium chloride solution with concentration of 36%; (5), electrolyzing the sodium chloride solution with concentration of 36% to obtain sodium hydroxide, hydrogen and chlorine, and illuminating hydrogen and chlorine to obtain hydrochloric acid; (6), adding quartz sand to sodium hydroxide for a reaction to generate sodium silicate; (7), adding hydrochloric acid to sodium silicate to prepare white carbon black again. By combined production and recycling, zero emission of water can be basically realized, production cost and environmental protection cost are greatly reduced, and the method is a good method for industrial production of the white carbon black.
Owner:江西双龙硅材料科技有限公司
Production process of electronic-grade hydrochloric acid
InactiveCN108609584AWill not cause excessiveAvoid pollutionChlorine/hydrogen-chloride purificationHydrogen chloride preparationHydrazine compoundDesorption
The invention discloses a production process of electronic-grade hydrochloric acid and relates to the technical field of chemical engineering. The production process of electronic-grade hydrochloric acid comprises the following steps: industrial-grade hydrochloric acid is placed in a reaction kettle, hydrazine hydrate is added to the reaction kettle, and the substances are stirred and mixed untilsufficient reaction; the material obtained after reaction is totally guided into a deacidification tower, negative pressure is maintained in the deacidification tower, air is introduced into the deacidification tower for bubbling stirring and blowing desorption of a solution, and escape gas and a tower bottom solution are obtained; the desorbed escape gas in the deacidification tower is compressedby a compressor; compressed escape gas enters an acid pickling tower for acid pickling, electronic-grade hydrochloric acid is obtained, non-condensable gas returns to the bottom of the deacidification tower for bubbling circulation, and negative pressure is maintained in the acid pickling tower. According to the production process of electronic-grade hydrochloric acid, new impurities are not introduced during reduction of free chlorine, waste gas or impurities produced in the production process is / are reasonably treated or applied, environmental pollution is avoided, and reasonable utilization of resources is realized.
Owner:成都市科隆化学品有限公司
Method of extracting bromine from bromine-bearing liquids or wastewaters
InactiveCN109081310AReduce concentrationLarge amount of processingBromineHydrogen chloride preparationBromineWastewater
The invention discloses a method of extracting bromine from bromine-bearing liquids or wastewaters. The method includes the steps of (1) acidifying a bromine-bearing liquid or wastewater, and introducing chlorine into the acidified liquid to oxidize bromine ions into free bromine so as to obtain an oxidized liquid; (2) feeding both the oxidized liquid and an extracting agent into a centrifugal extractor to carry out counter-current extraction so as to obtain extract and raffinate; (3) feeding both the raffinate and a stripping agent solution into the centrifugal extractor to carry out counter-current stripping so as to obtain hydrobromic acid-bearing absorption liquid and the extracting agent; (4) feeding the absorption liquid into a distilling column, introducing chlorine into the absorption liquid, generating hydrogen chloride and free bromine by reaction of the chlorine with hydrobromic acid in the absorption liquid, allowing the hydrogen chloride to turn into hydrochloric acid uponcontact with water, and distilling the free bromine during distilling to obtain finished bromine. The method herein is continuous, high in automation level, high in establishment speed of phase balance, and low in extracting agent consumption.
Owner:山东省海洋化工科学研究院
DCS control method for gas supply process in hydrogen chloride production
ActiveCN106406255ATimely mediationStable mediationHydrogen chloride preparationProgramme total factory controlAutomatic controlControl system
The invention relates to a DCS control method for a gas supply process in hydrogen chloride production. In hydrogen chloride production, hydrogen chloride gas produced in a synthetic furnace is exported from the bottom of a cooler of the synthetic furnace through a hydrogen chloride outlet pipe, and enters a hydrogen chloride distributing table, the hydrogen chloride gas supply process adopts a DCS control mode, and a one-key gas supply start button is arranged on a display device of hydrogen chloride DCS control system operator station. The invention provides the DCS control method for one-key completion of the gas supply process in the hydrogen chloride production, and the method is simple and convenient to operate, high in degree of automation and safety factor, and good in stability, thereby truly realizing automated control of the hydrogen chloride gas supply process, the labor intensity is reduced, and the production cost is also reduced.
Owner:TIANNENG CHEM
High-purity hydrochloric acid production process
The invention discloses a high-purity hydrochloric acid production process. The high-purity hydrochloric acid production process comprises the steps that chlorine gas and hydrogen gas are burnt on a combustion table of a synthesis furnace to generate white hydrogen chloride gas, and water in a cooling tower absorbs the hydrogen chloride gas to generate hydrochloric acid with the concentration of 31%; a sodium sulfite solution is dripped from the top of a hydrochloric acid storage tank through a dropping tank and fully reacted with free chlorine; and the processed hydrochloric acid solution enters a resin tower, and after the tower is filled with the acid liquid, excess iron is removed by adsorption to obtain high-purity hydrochloric acid which meets the production requirements. The high-purity hydrochloric acid production process can effectively reduce the content of the free chlorine and the iron in the hydrochloric acid, so that the prepared high-purity hydrochloric acid can meet theprocess condition requirements of a membrane process for producing alkali, the service life of a subsequent reaction device is prolonged, the economic benefit of a company is increased, and obvious advantages are achieved.
Owner:张凤芹
Method and system for producing high-purity hydrogen chloride
InactiveUS20130259796A1Simple and easy mannerSimple processDispersed particle separationLiquid-gas reaction processesHydrogenExcess molar quantity
The present invention provides a method for producing high-purity hydrogen chloride, comprising the steps of: purifying each of crude hydrogen and crude chlorine as raw materials to a purity of 99.999% or higher; reacting an excessive molar amount of the purified hydrogen with the purified chlorine at a temperature ranging from 1,200° C. to 1,400° C. to synthesize hydrogen chloride; converting the hydrogen chloride to a liquid state by compression; and purifying the hydrogen chloride and separating unreacted hydrogen by fractional distillation. The invention also provides a system for carrying out the method. According to the method and system, an environmentally friendly production process can be provided, which can easily produce a large amount of hydrogen chloride having a purity of 3 N (99.9%)-6 N (99.9999%) in a cost-effective manner and enables energy consumption to be significantly reduced.
Owner:HONG IN CHEM +1
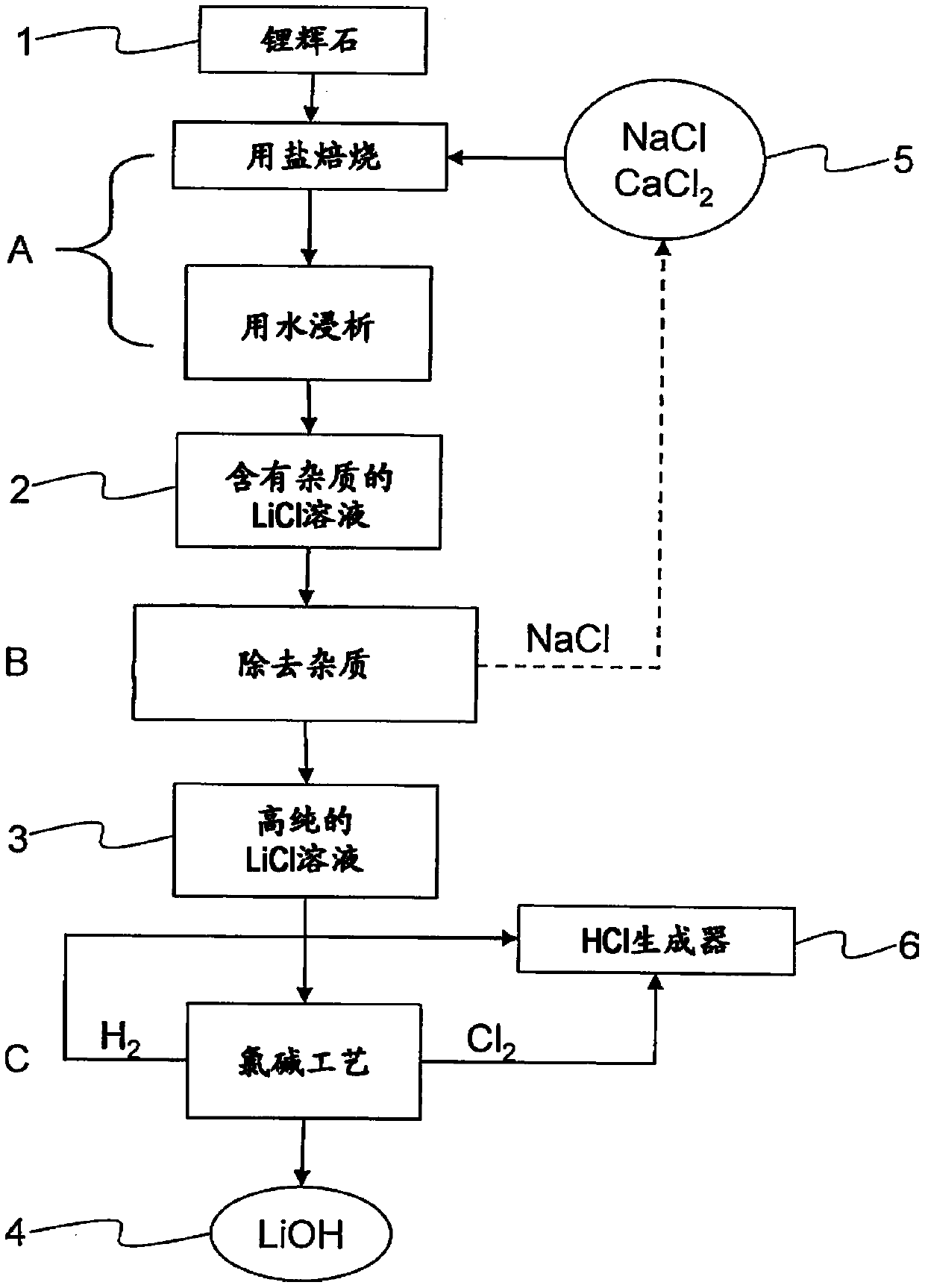
Method for producing lithium hydroxide from lithium-containing ore
InactiveCN110494573ACalcium/strontium/barium carbonatesElectrolysis componentsMetal chlorideLithium oxide
The invention relates to a method for producing lithium hydroxide (4), in particular highly pure lithium hydroxide, for use in batteries and / or accumulators, from lithium-containing ore and / or mineraland / or from lithium-containing earths (1) by means of a chlor-alkali process. The aim of the invention is to create a solution which, in the case of such a method, increases the extraction rate of highly pure lithium hydroxide in the application of a chlor-alkali process. This aim is achieved in that, in a calcining and leaching step (A), a lithium chloride solution (2) is produced, the lithium-containing ores and / or minerals and / or earths (1) first being roasted, one or more metal chlorides (5) and / or a mixture of metal chlorides (5) being used, and then leached out, water being used, then ahighly pure lithium chloride solution (3) being produced in a subsequent purification step (B), the lithium chloride solution (2) being purified in particular by removing cations, such as sodium, potassium, calcium, magnesium, and / or iron, from the lithium chloride solution (2), and then lithium hydroxide (4), in particular highly pure lithium hydroxide, being produced in a subsequent electrolysis step (C), the highly pure lithium chloride solution (3) being subjected to a membrane electrolysis process, which produces chlorine gas and hydrogen as byproducts.
Owner:SMS GRP GMBH
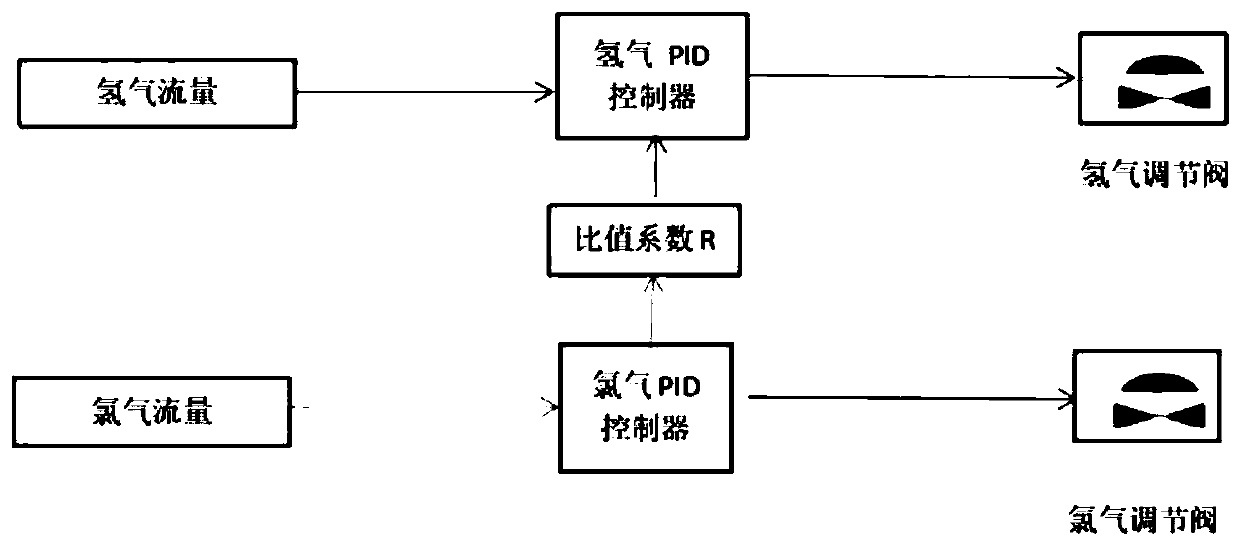
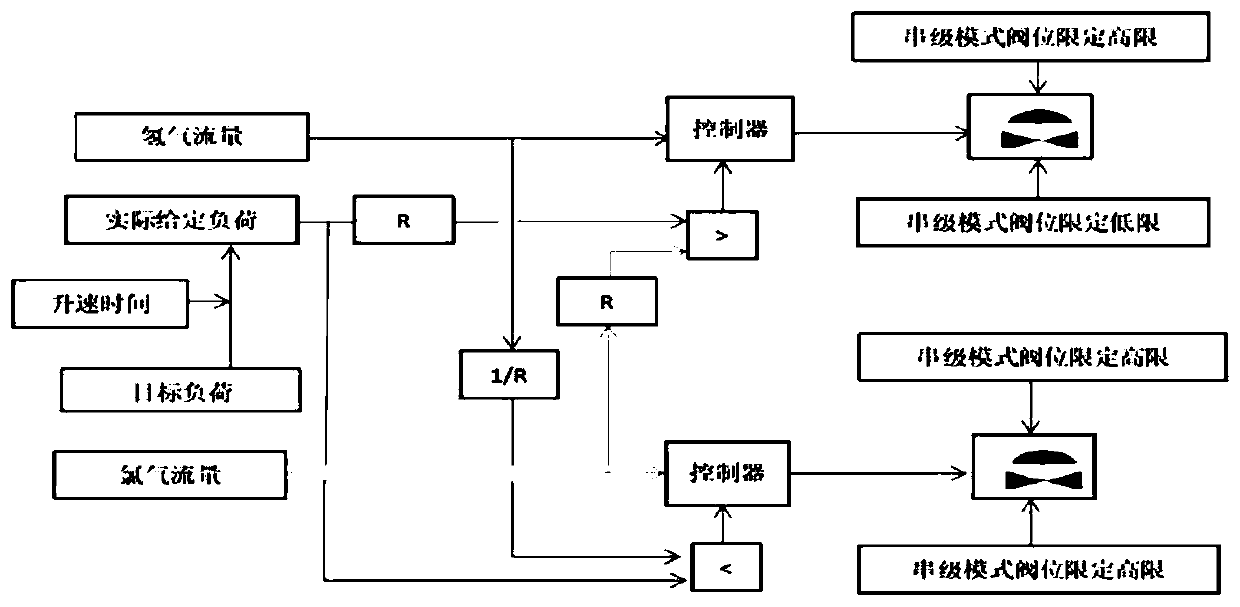
Method and system for automatic chlorine and hydrogen proportioning control of hydrogen chloride synthesis furnace, and synthesis furnace
ActiveCN110589769AGuaranteed uptimeRealize unattendedHydrogen chloride preparationLower limitAutomatic control
The invention provides a method and system for automatic chlorine and hydrogen proportioning control of a hydrogen chloride synthesis furnace, and the synthesis furnace. The flow rate of chlorine is limited through low-level selection; the flow rate of hydrogen is limited through high-level selection; when the gas flow rate of hydrogen fluctuates and the fluctuation amount is lower than a first hydrogen set threshold value, or when the gas flow rate of chlorine fluctuates and the fluctuation amount is lower than a first chlorine set threshold value, the hydrogen flow rate and the chlorine flowrate are mutually sensed and locked through high-level selection and low-level selection, so that when load adjustment and flow disturbance occur, excessive ratio of hydrogen is ensured, the ratio ofhydrogen is larger than a safe low limit, and generation of free chlorine is avoided. Through adoption of a control strategy based on the cross restriction principle, the function of automatically adjusting the chlorine-hydrogen ratio of the synthesis furnace is realized, and remote automatic control of the chlorine-hydrogen flow ratio of the hydrogen chloride synthesis furnace is realized undera condition of ensuring safe production, so that hidden dangers of on-site safety risks are avoided, and the purposes of automatic people reduction and people substitution can be realized.
Owner:DEZHOU SHIHUA CHEM
Process and device for preparing fumed silica and activated carbon at low temperature with rice hull ash as raw material
ActiveCN106219556ASolve high temperature processSolve the dangerSilicaHydrogen fluorideActivated carbonFumed silica
The invention provides a process for preparing fumed silica and activated carbon at low temperature with rice hull ash as a raw material. In the process, rice hull ash and other kinds of waste are used as the raw material and react with fluorine-containing acid liquor, hydrochloric acid and nitric acid to prepare high-value-added products fumed silica and activated carbon, the content of SiO2 in the prepared products is larger than 99.9%, the specific area measured through a multi-point BET method is 350-500 m<2> / g, and the effect of turning waste into wealth is achieved. Acidic gas generated in the preparation process is absorbed through a negative pressure water absorbing tank to prepare corresponding acidic liquid, and when concentrated to certain concentration, the acidic liquid can be used circularly. The invention further provides a production device used in the process, parts used in the production device are common tools used in a chemical plant and low in cost, and large-scale industrial production can be achieved.
Owner:HUANGGANG NORMAL UNIV +1
Preparation method of high-purity hydrogen chloride
ActiveCN110697656AExtended service lifeEasy to useChlorine/hydrogen-chloride purificationOther chemical processesSorbentPhysical chemistry
The invention relates to the field of fine chemical industry, and in particular relates to a preparation method of high-purity hydrogen chloride. Compared with the prior art, the preparation method ofthe high-purity hydrogen chloride uses a styrene / ionic liquid high-crosslinking adsorbent which has a better effect of adsorbing hydrogen chloride impurities, and effectively reduces the impurity content of product gas; moreover, a deep separation type gas purifier is added, and tests show that the water content of the prepared high-purity hydrogen chloride can be reduced to 100 ppb or less and the gas impurities can be reduced to 50 ppb or less; moreover, compared with the prior separation type gas filter technology, the process is enlarged, the flow rate can reach 400 standard liters / minuteat the highest, and the production efficiency is greatly improved.
Owner:ZHEJIANG BRITECH CO LTD
Method of producing hydrogen chloride
InactiveCN103708420AAvoid temperature riseSuppression temperatureEnergy based chemical/physical/physico-chemical processesHydrogen chloride preparationHydrogenPhysical chemistry
The invention relates to a method of producing hydrogen chloride. The purpose of the invention is to provide a method of producing hydrogen chloride that can inhibit temperature rising of a reaction system through more simple steps. According to the producing method, hydrogen and chlorine are irritated in the existence of hydrogen chloride gas, and the hydrogen chloride gas is generated because of reaction between hydrogen and chlorine.
Owner:SUMITOMO SEIKA CHEM CO LTD
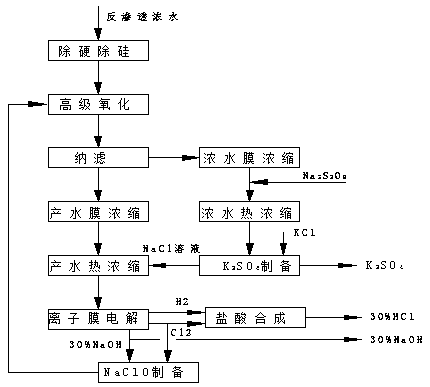
Method for realizing zero discharge and resource utilization of reverse osmosis concentrated water in coal chemical industry
ActiveCN111018230ARealize resource utilizationEliminate the crystallization processWater contaminantsMultistage water/sewage treatmentReverse osmosisWater production
The invention discloses a method for realizing zero discharge and resource utilization of reverse osmosis concentrated water in coal chemical industry. The method comprises the following steps: removing hardness and silicon from reverse osmosis concentrated water, carrying out advanced oxidation on the effluent to remove organic matters, feeding into a nanofiltration system, and separating salts to obtain sodium sulfate concentrated water and sodium chloride produced water; concentrating the sodium sulfate concentrated water by a concentrated water membrane, adding an oxidant, carrying out concentrated water hydrothermal concentration treatment, and carrying out potassium sulfate preparation treatment on the effluent to obtain a potassium sulfate product and a sodium chloride solution; andcarrying out water production membrane concentration treatment on the sodium chloride produced water, carrying out produced water thermal concentration treatment, and feeding the effluent into an ionic membrane electrolysis unit to obtain H2, Cl2 and a 30% NaOH solution, wherein H2 and part of Cl2 are used for preparing a hydrochloric acid solution, and part of Cl2 and part of the NaOH solution enter a NaClO preparation unit to prepare NaClO. According to the method, reverse osmosis concentrated water is pretreated to prepare the approximately saturated sodium chloride and sodium sulfate concentrated salt water without crystallization so as to reduce the treatment cost, and finally the concentrated salt water is prepared into acid-alkali and potassium sulfate with industrial values, so that resource utilization is realized, and the method has good application prospect.
Owner:BEIJING CYCLE COLUMBUS ENVIRONMENTAL SCI & TECH
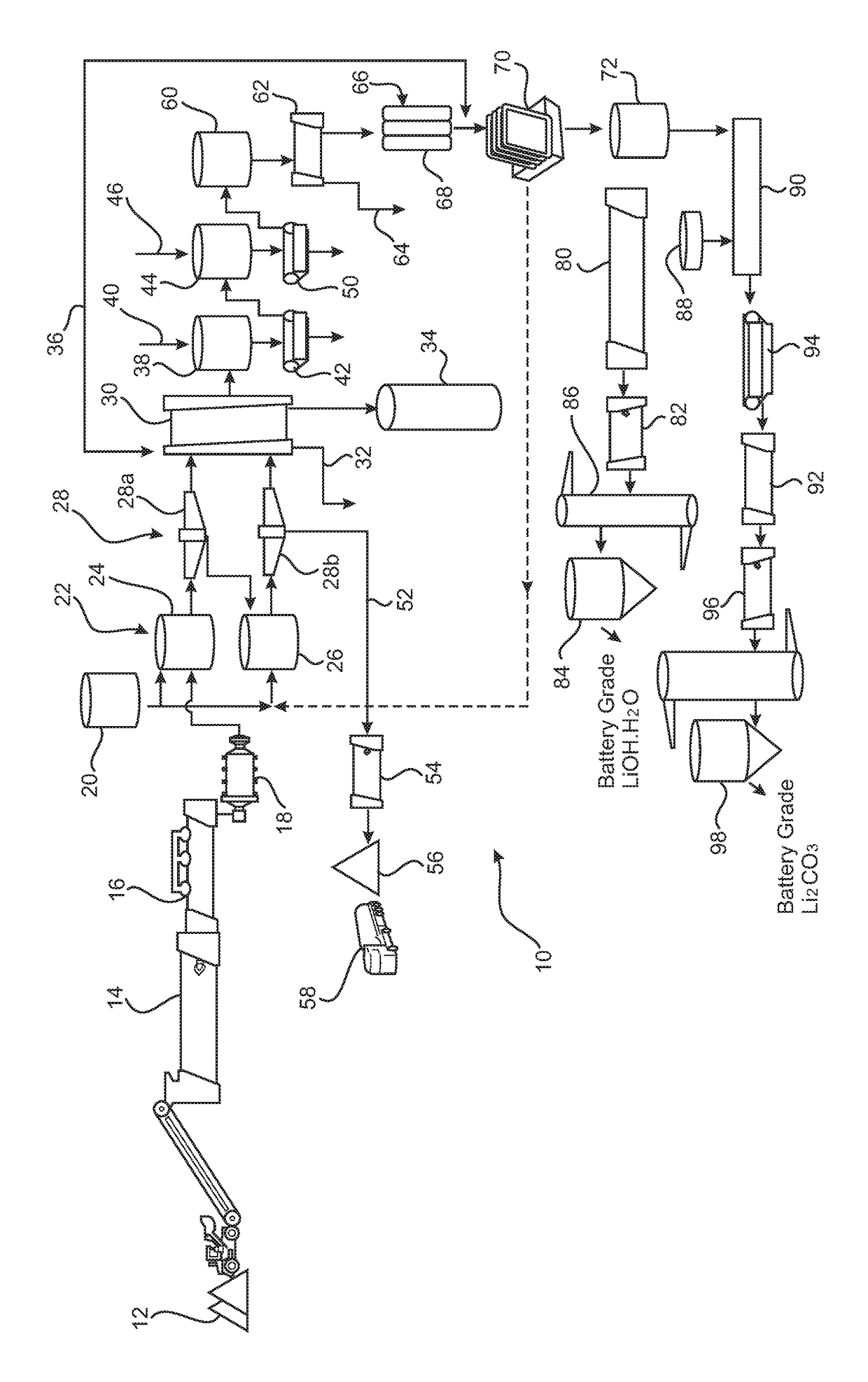
Processing of lithium containing material
A process (10) for the treatment of a lithium containing material, the process comprising the steps of:(i) Preparing a process solution from the lithium containing material (12);(ii) Passing the process solution from step (i) to a series of impurity removal steps (36) thereby providing a substantially purified lithium chloride solution; and(iii) Passing the purified lithium chloride solution of step (ii) to an electrolysis step (70) thereby producing a lithium hydroxide solution.
Owner:REED ADVANCED MATERIALS PTY LTD
Apparatus for HCl synthesis with steam raising
What is described is an apparatus for performance of a process for hydrogen chloride synthesis from chlorine and hydrogen or from chlorine and hydrocarbons with integrated heat recovery, wherein the combustion chamber and the heat exchanger are arranged in the steam drum of a shell boiler which works according to the waste heat boiler principle.
Owner:SGL CARBON SE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com