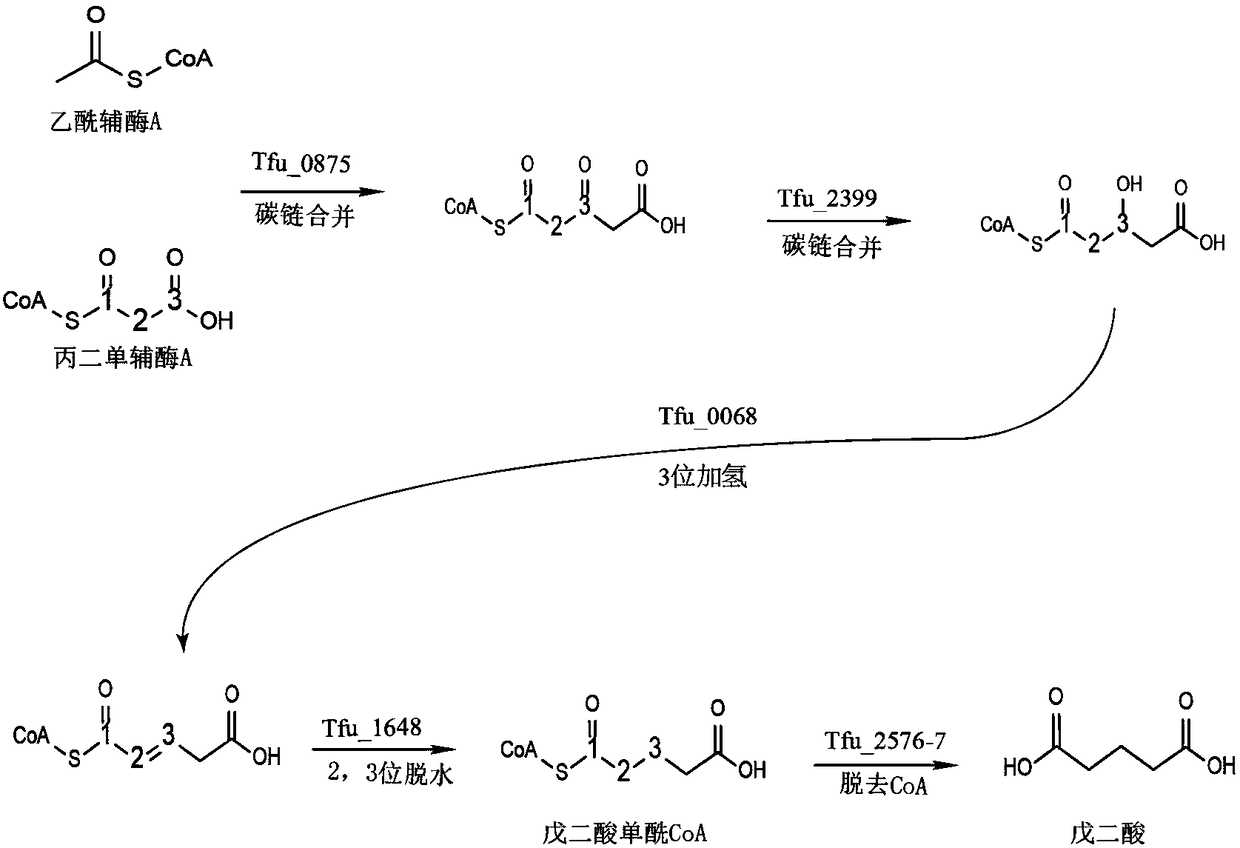
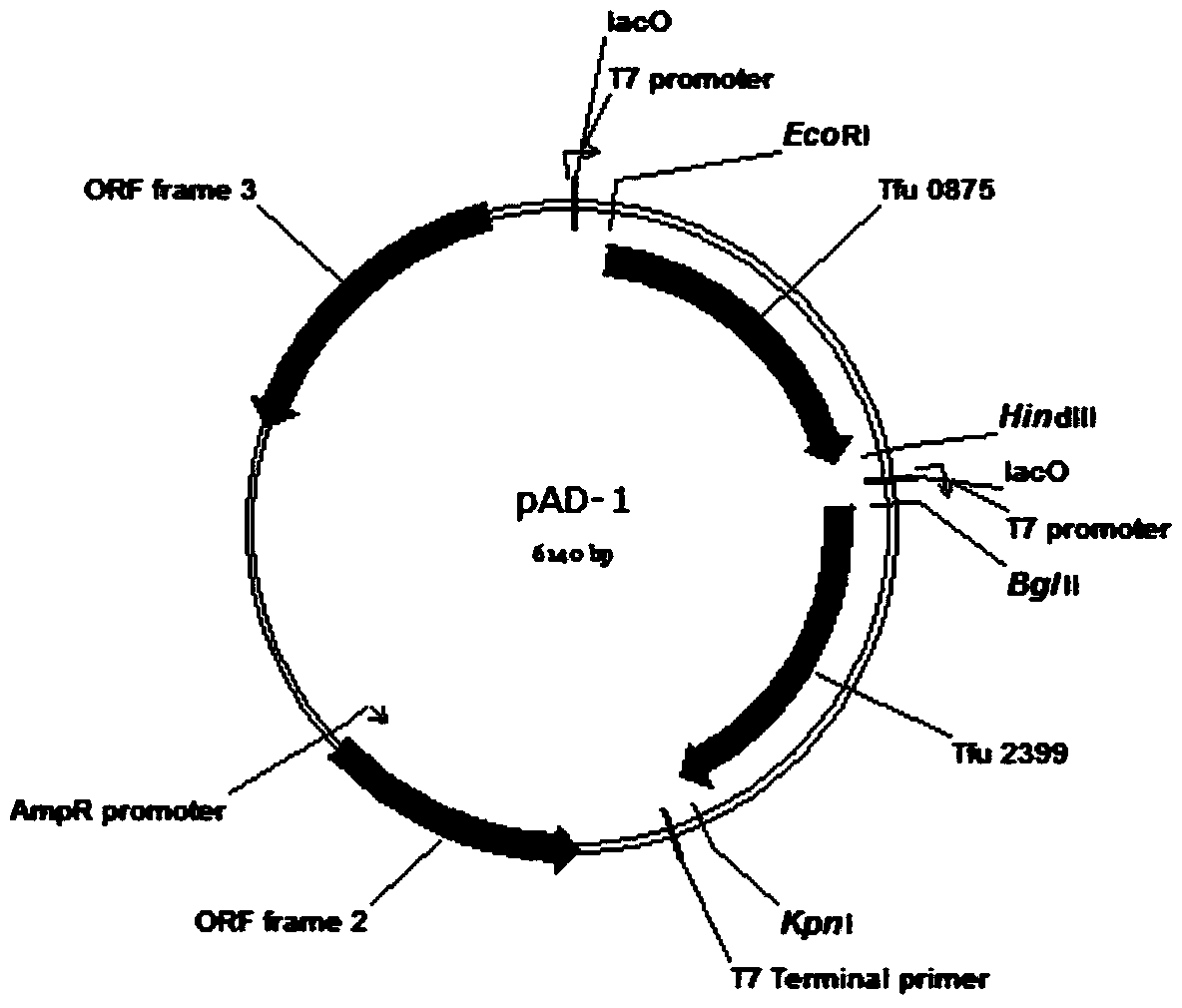
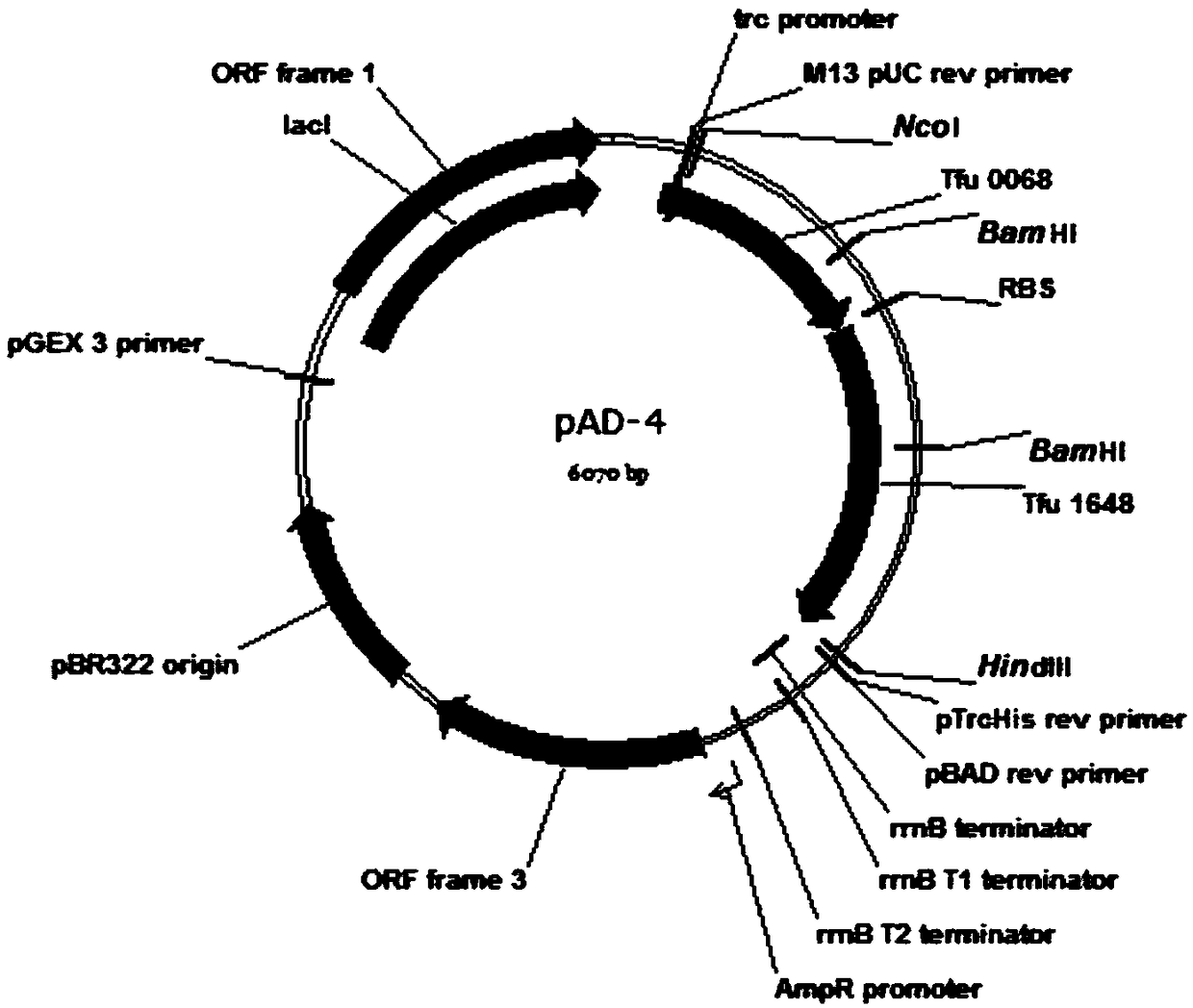
Total biosynthesis method of glutaric acid
A technology for producing glutaric acid and genes, applied in the field of bioengineering, can solve the problems of unsuitability for large-scale production, high cost, low yield, etc., and achieves the effects of low cost, convenient operation and reduced pollution degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Construction of recombinant plasmid pAD-1 and acquisition of recombinant Escherichia coli.
[0033] The sequences of Tfu_0875, Tfu_2399, Tfu_0068, Tfu_1648, Tfu_2576, Tfu_2577 are described in NCBI.
[0034] Plasmid pRSFDuet-1 was double digested with EcoR I and HindIII, the target gene fragment (3798bp) was recovered by cutting the gel, and the plasmid pUC57-Tfu_0875 was digested with the same enzyme, the target gene fragment Tfu_0875 was recovered by cutting the gel, and then the two target fragments were purified with T 4 DNA ligase ligation, transformation of JM109, positive transformants were picked by colony PCR, and the extracted plasmid was digested for verification. The verified plasmid was named pRSF-Tfu_0875. Digest the plasmid pRSF-Tfu_0875 with Bgl II and Kpn I, cut the gel to recover the 4936bp target gene fragment, digest the plasmid pUC57-Tfu_2399 with the same enzyme, cut the gel to recover the target gene fragment, and then separate the two ...
Embodiment 2
[0037] Example 2: Shake flask fermentation and result analysis of recombinant Escherichia coli.
[0038] Fermentation medium:
[0039] SOB medium, the composition is 2% tryptone + 0.5% yeast powder + 0.05% NaCl + 2.5mM KCl + 10mM MgCl 2 +8g / L glucose+50μg / ml kanamycin sulfate+50μg / ml ampicillin+50μg / ml streptomycin.
[0040] Seed solution preparation: Streak the strains preserved in glycerol on a plate, pick a single colony and inoculate it in a 250ml Erlenmeyer flask filled with 50ml of LB liquid medium, shake the flask overnight at 37°C and 250rpm / min.
[0041] Fermentation conditions: 2% inoculum size (1ml), inoculated in shake flask fermentation medium to make initial OD 600 0.1. Cultivate to OD at 37℃, 250r / min 600 Add 1.0mM IPTG to induce the recombinant bacteria at 0.6, respectively, and change to 30°C, 250rpm / min culture.
[0042] Result analysis: take a sample every 4 hours during the fermentation process, centrifuge at 10,000r / min for 2 minutes to separate the fe...
Embodiment 3
[0043] Example 3: Gas-phase mass spectrometry detection and result analysis of glutaric acid.
[0044] The fermented samples were first frozen in a -80°C refrigerator for 2-3 hours to ensure complete freezing, and then placed in a freeze dryer for 2 days until completely dry. Take out the completely dry sample, add 3ml of distilled water, and ultrasonically dissolve the insoluble matter. After complete dissolution, add 3ml of ethyl acetate to extract for 2 minutes. After static layering, transfer the ethyl acetate layer to another glass reaction test tube. If the emulsification phenomenon is serious and the layers cannot be separated, transfer it to a glass centrifuge tube and centrifuge at 10,000r / min. 2 min; the lower layer was continuously extracted twice with 5 ml of ethyl acetate, all the ethyl acetate layers were collected, and dried under nitrogen at 30°C. Add 0.5ml of acetonitrile to the reaction tube of the dried extract, ultrasonically dissolve, then add 0.5ml of si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com