PH responsive polymer carrier, micelle prepared from same, preparation method and application
A technology of polymers and mixed micelles, which is applied in the directions of drug combinations, pharmaceutical formulations, and medical preparations with non-active ingredients, etc., can solve the problem of increased CMC value of mixed micelles, large drug release amount, and reduced drug target release ability. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
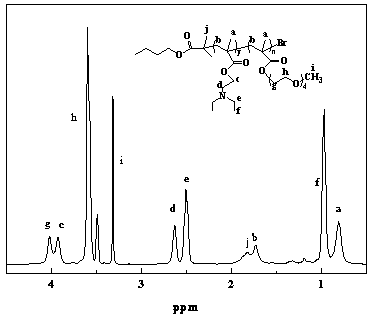
Embodiment 1
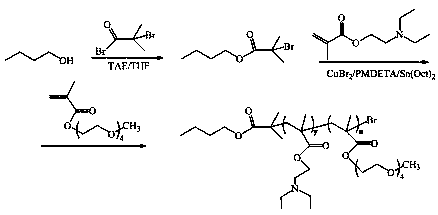
[0068] The preparation process of diblock copolymer A (PDEAEMA-PPEGMA):
[0069] (1) Synthesis of small molecule initiator: Weigh n-butanol (4.575ml, 0.05mmol) and add it into a dry 250mL anhydrous and anaerobic reaction bottle together with the solvent, seal it with a reverse rubber stopper, vacuum-pass After 3 times of nitrogen, under the protection of nitrogen, add the dehydrated solvent dichloromethane (50mL) and dehydrated triethylamine (TEA, 6.95mL) successively with a syringe, then cool to 0°C in an ice bath, and stir Slowly add 2-bromoisobutyryl bromide (6.183mL) drop by drop under the conditions. After the dropwise addition, react at 0°C for 2h, then raise the temperature to 30°C, and continue the reaction for 12h. After the reaction, use dilute hydrochloric acid and pure water respectively Wash three times, drop the organic phase into 0°C n-hexane of ten times its volume to precipitate, filter, and finally vacuum-dry at 40°C for 48 hours;
[0070] (2) Synthesis of P...
Embodiment 2
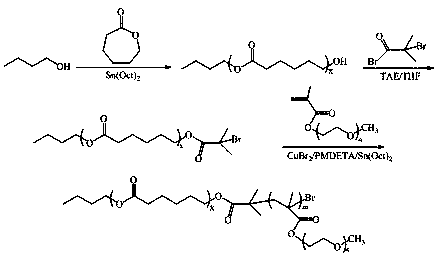
[0073] The preparation process of diblock copolymer B (PCL-PPEGMA):
[0074] (1) Synthesis of PCL-OH: Lactone monomer ε-CL (10g), n-butanol (0.1482g), initiator Sn(Oct) 2 (78.6mg) and solvent were added to a 100mL anhydrous and oxygen-free reaction bottle, sealed with a reverse rubber stopper, vacuumed-nitrogen three times, and reacted in an oil bath at 130°C for 24h under the protection of nitrogen. After the reaction, the organic phase Slowly add dropwise to 200mL cold aqueous methanol solution (v / v=1:1) to precipitate, filter, and finally vacuum-dry at 45°C for 48h, Mn=6000, PDI=1.2;
[0075] (2) Synthesis of brominated polycaprolactone (PCL-Br): Weigh PCL-OH (6g, Mn=6000) and add it into a dry 100mL anhydrous anaerobic reaction bottle together with the solvent, and use a reverse rubber stopper Carry out sealing, after evacuation-nitrogen 3 times, under the protection of nitrogen, add the solvent dichloromethane (30mL) that removes water and the triethylamine (TEA, 0.3mL) ...
Embodiment 3
[0079] With reference to the preparation process of diblock copolymer A in embodiment 1, PDEAEMA is prepared 35 -PPEGMA 13 , the specific process is as follows: pH responsive monomer DEAEMA (7.5g), small molecule initiator (0.233g), catalyst CuBr 2 (2.78mg) and solvent were added to a 150mL anhydrous and oxygen-free reaction bottle, sealed with a reverse rubber stopper, vacuumed-nitrogen three times, and under the protection of nitrogen, THF (35mL) dewatered was added successively with a syringe, and prepared Body PMDETA (69.2mg) and reducing agent Sn (Oct) 2 (243 mg), after freezing in liquid nitrogen, vacuumize and blow nitrogen three times. After thawing, stir for 15 minutes and then start to heat up. After reacting in an oil bath at 65°C for 5 hours, add monomer PEGMA (4 g, Mn=300) and continue the reaction for 24 hours. After the reaction, the solution obtained was passed through a neutral alumina chromatography column to remove the catalyst, and then most of the THF wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com