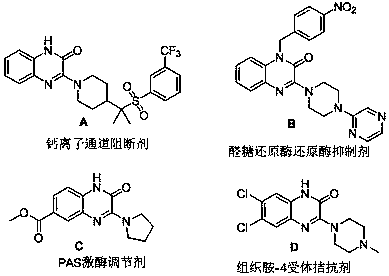
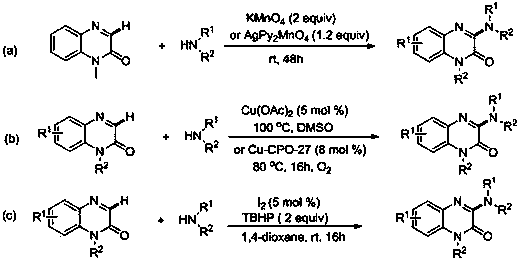Photocatalytic preparation method of 3-aminoquinoxaline-2(1H)-ketone compound
A ketone compound and photocatalytic technology, applied in the direction of organic chemistry, can solve the problems of difficult to deal with metal ion residues, poor compatibility of reactive functional groups, high reaction temperature and other problems, and achieve the goal of reducing metal pollution, good compatibility and clean energy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] At room temperature, in a 15mL reaction tube, sequentially add quinoxalin-2(1H)-one 1a (0.2mmol), morpholine (0.4mmol), water-soluble eosin (0.002mmol), and tetrahydrofuran 2mL, mix well, and then Under the irradiation of 3W watt blue LED lamp, the reaction was stirred for 12h. After the reaction was detected by TLC, 2 mL of water was added, followed by extraction with ethyl acetate for 3 times, the extracts were combined, dried over anhydrous sodium sulfate, and then concentrated under vacuum (0.08Mpa) to nothing. Solvent, to obtain the crude product, then wash with the mixed eluent of sherwood oil and ethyl acetate with a volume ratio of 3:1, silica gel column flash column chromatography, obtain the present embodiment 3-aminoquinoxaline-2 (1H) - The ketone product 3aa is 40.2 mg of white solid, and the yield is 82%.
[0035] The obtained product nuclear magnetic spectrum data are: 1 H NMR (CDCl 3 , 500 MHz, ppm): δ 7.57 (dd, J =1.3Hz, 7.7Hz, 1H), 7....
Embodiment 2
[0038]
[0039] At room temperature, in a 15mL reaction tube, sequentially add quinoxalin-2(1H)-one 1b (0.2mmol), morpholine (0.4mmol), water-soluble eosin (0.002mmol), and tetrahydrofuran 2mL, mix well, and then Under the irradiation of 3W (watt) blue LED lamp, the reaction was stirred for 18h. After the reaction was detected by TLC, 2 mL of water was added, followed by extraction with ethyl acetate for 3 times, the extracts were combined, dried over anhydrous sodium sulfate, and then concentrated under vacuum (0.08Mpa) to nothing. Solvent, to obtain the crude product, then wash with the mixed eluent of sherwood oil and ethyl acetate with a volume ratio of 3:1, silica gel column flash column chromatography, obtain the present embodiment 3-aminoquinoxaline-2 (1H) - The ketone product 3ba is 39.4 mg of white solid, with a yield of 67%.
[0040] The obtained product nuclear magnetic spectrum data are: 1 H NMR (CDCl 3 , 500 MHz, ppm): δ 7.56 (dd, J =1.4Hz,7.8Hz, 1H), 7....
Embodiment 3
[0043]
[0044] At room temperature, in a 15mL reaction tube, add quinoxalin-2(1H)-one 1c (0.2mmol), morpholine (0.4mmol), rose bengal (0.002mmol), and tetrahydrofuran 2mL in sequence, mix well, and then Under the irradiation of 3W blue LED light, the reaction was stirred for 12h. After the reaction was detected by TLC, 2 mL of water was added, followed by extraction with ethyl acetate for 3 times, the extracts were combined, dried over anhydrous sodium sulfate, and then concentrated under vacuum (0.08Mpa) to nothing. Solvent, to obtain the crude product, then wash with the mixed eluent of sherwood oil and ethyl acetate with a volume ratio of 3:1, silica gel column flash column chromatography, obtain the present embodiment 3-aminoquinoxaline-2 (1H) - The ketone product 3ca is 45.6 mg of white solid, with a yield of 71%.
[0045] The NMR data of the obtained product are . 1 H NMR (CDCl 3 , 500 MHz, ppm): δ 7.56 (dd, J =1.4 Hz, 7.9 Hz, 1H), 7.30 (t, J = 7.1 Hz, 2H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com