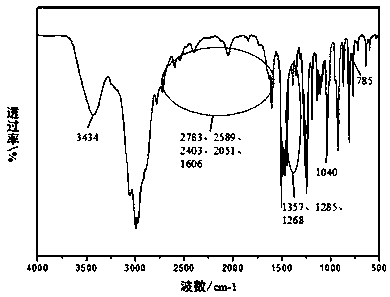
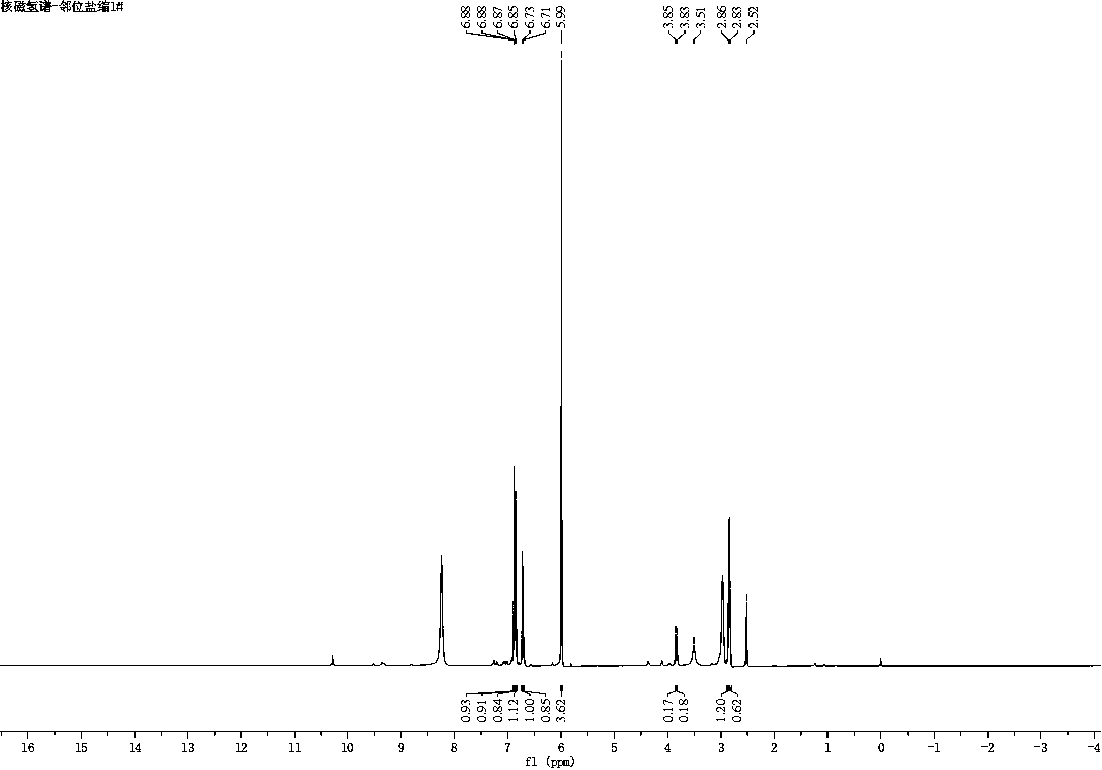
Method for preparing berberrubine from o-vanillin as raw material
A technology of ortho-vanillin and berberyrine, which is applied in the field of preparation of berberyrine, can solve the problems of expanding the use of unfavorable berberyrine drugs, increasing production costs, and limiting the production of berberyrine. wide, time-saving and quality-guaranteed effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
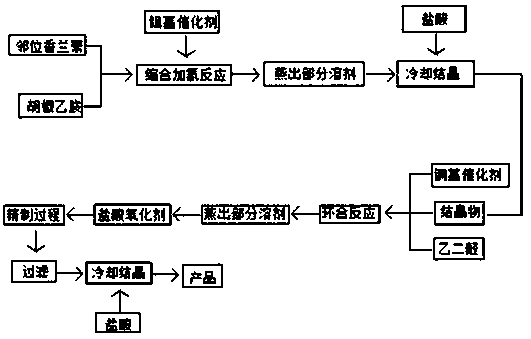
[0039] (1) Weigh a certain amount (molar ratio) of piperonyl ethylamine and o-vanillin into a 250ml autoclave, stir and heat up to 70°C, distill under reduced pressure (pressure 0.095Mp), continue to heat up to 95°C, no Evaporation of water is the end point of the reaction. After 30 minutes of heat preservation, the preparation of the Schiff base is completed.
[0040] (2) Add 0.25g of nickel-based catalyst to the autoclave, blow nitrogen to discharge the air in the autoclave, and raise the temperature to 70°C, increase the pressure to 4Mp, and raise the temperature to 115°C under the hydrogen pressure of 3Mp in the autoclave, and close the hydrogen valve. Up to 4Mp until the pressure indication is no longer falling, keep the temperature at 4Mp, hold the pressure for 50min, cool down and stand still, and obtain the condensed hydrogenated liquid.
[0041] (3) Cool down the condensation and hydrogenation solution to 75°C, start to evaporate the solvent at room temperature (recov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com