Application of polyguluronic acid propyl sulfate in preparation of anticoagulant medicine
The technology of propyl guluronic acid and polyguluronic acid is applied in the application field of poly propyl guluronic acid sulfate in the preparation of anticoagulant drugs, and can solve the problem of strong side effects of anticoagulant drugs. , easy pollution and other problems, to achieve significant anticoagulant effect, less toxic and side effects, and enhance the effect of the fibrinolytic system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
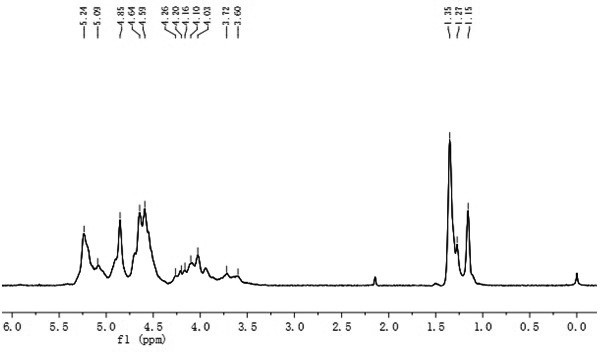
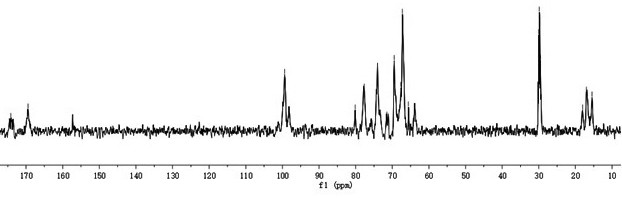
[0023] Embodiment 1: the preparation of polyguluronic acid propyl ester sulfate
[0024] The invention uses polyguluronic acid (Mw=12.83kDa, purity 80.0%) as a starting material, and prepares polyguluronic acid propyl sulfate through propyl esterification and sulfate esterification.
[0025] The preparation method of the polyguluronic acid propyl ester sulfate is as follows: weigh 10.00 g of polyguluronic acid, add 5.00 mL of ethanol-water mixed solution, stir evenly, and swell overnight. After adding 40.00mL of 1,2-propylene oxide (density 0.83g / mL) into the reaction flask, stir and add 0.08g NaOH, add 10.00g of polyguluronic acid swollen overnight, and reflux at 36°C Reaction 8h. After the reaction, the precipitate was washed three times with 85% ethanol solution, and the obtained sandy precipitate was vacuum-dried overnight to obtain polypropyl guluronate.
[0026] In an ice bath, slowly drop 16.00 mL of chlorosulfonic acid (density 1.77 g / mL) into 70.79 mL of anhydrous f...
Embodiment 2
[0033] Example 2: Effect of polyguluronic acid propyl sulfate on mouse coagulation time (CT) and bleeding time (BT)
[0034] Forty male Kunming mice of 18-22 g were randomly divided into blank group, heparin group, PSS group and PGGS group, with 10 mice in each group. Adaptive feeding for 7 days. All were administered by intraperitoneal injection, and the administration volume was 0.1mL / 10g. The blank group was given the same amount of normal saline, the heparin group was given a dose of 15 mg / kg, and the doses of the PSS group and the PGGS group were both 28 mg / kg. kg. After 30 minutes of administration, a capillary glass tube was inserted into the venous plexus of the mouse's inner canthus to take blood, and the time from blood collection to the appearance of coagulation filaments was recorded as the index of testing coagulation time (CT); Transect, and start timing to record the time from the natural outflow of blood to the natural cessation of blood, which is the indicat...
Embodiment 3
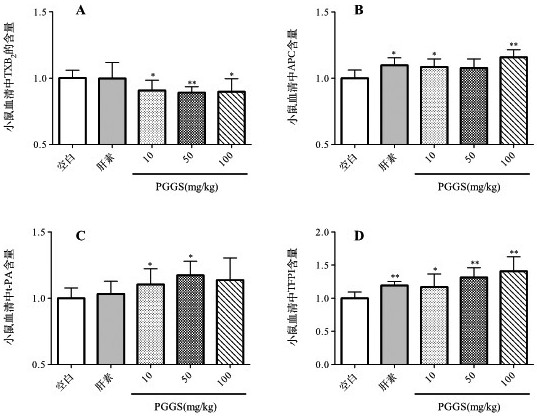
[0039] Example 3: Effect of polyguluronic acid propyl ester sulfate on platelet aggregation
[0040] The effect of polyguluronic acid propyl sulfate on platelet aggregation was evaluated by ADP-induced platelet aggregation in mouse plasma.
[0041] Get 84 male Kunming mice of 22-26g, be randomly divided into blank group, clopidogrel group, heparin group, PGGS administration group (different dosage, low dose 10mg / kg, middle dose 50mg / kg, high dose 100mg / kg) 12 in each group. Adaptive feeding for 3 days. The clopidogrel group was administered by intragastric administration, and the rest were administered by intraperitoneal injection, with an administration volume of 0.1 mL / 10 g, for 7 consecutive days. 30 minutes after the last administration, the eyeballs were picked to take blood, and immediately mixed with sodium citrate at a ratio of 1:9, and then centrifuged at 3000rpm for 10 minutes to prepare plasma. ADP was used as an inducer to measure the platelet aggregation rate. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com