Cabazitaxel-oligo/polylactic acid conjugated prodrug, preparation, preparation method and application thereof
A technology of cabazitaxel and polylactic acid, which is used in preparations and preparations, and the field of cabazitaxel-oligo/polylactic acid conjugated prodrugs, can solve the problems of affecting application, poor water solubility, etc., and achieve good anti-tumor, reduce damage, The effect of good clinical transformation function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
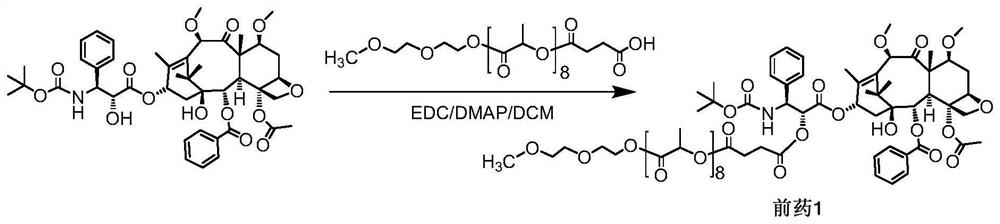
[0052] Example 1o (LA) 8 - Synthesis of CTX-coupled prodrug 1, as figure 1 Shown:
[0053] Add CTX (cabazitaxel, 100mg, 0.1196mmol), mPLA600·SA (129.2mg, 0.1794mmol) and DMAP (21.9mg, 0.1794mmol) sequentially into a 100ml round bottom flask equipped with a spherical condenser, dissolve in 4ml water, dichloromethane, and EDC (27.9 mg, 0.1794 mmol) was added rapidly dropwise. Stir at 43°C overnight, and observe the reaction by thin-layer chromatography (developing solvent: DCM:MeOH=20:1). When the reaction was basically completed, the reaction liquid was cooled, and then washed with 5% citric acid, saturated sodium bicarbonate, and saturated saline respectively. The organic layer was dried over anhydrous sodium sulfate, and filtered after complete drying. The filtrate was rotary evaporated to remove the solvent. The final product 1 (corresponding to the structure of n=8 in formula I, 168.3 mg, yield 91.5%) was isolated and purified by column chromatography (DCM:MeOH=80:1). ...
Embodiment 2
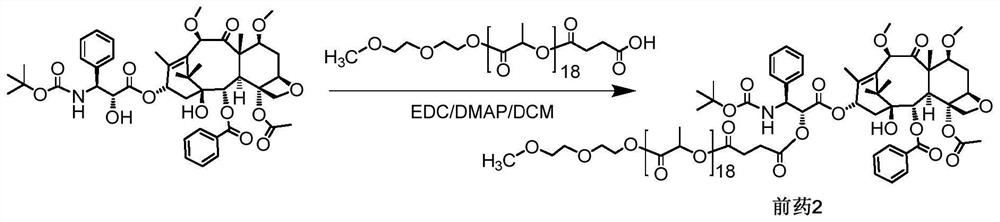
[0056] Example 2o(LA) 18 - Synthesis of CTX-coupled prodrug 2, as figure 2 Shown:
[0057] Add CTX (200mg, 0.2393mmol), mPLA1200·SA (560mg, 0.3590mmol) and DMAP (43.9mg, 0.3590mmol) successively in a 100ml round bottom flask equipped with a spherical condenser, dissolve in 7ml of anhydrous dichloromethane, EDC (55.7mg, 0.3590mmol) was added rapidly dropwise. Stir at 43°C overnight, and observe the reaction by thin-layer chromatography (developing solvent: DCM:MeOH=20:1). When the reaction was basically completed, the reaction liquid was cooled, and then washed with 5% citric acid, saturated sodium bicarbonate, and saturated saline respectively. The organic layer was dried over anhydrous sodium sulfate, and filtered after complete drying. The filtrate was rotary evaporated to remove the solvent. The final product 2 (corresponding to the structure of n=18 in formula I, 475.7 mg, yield 83.6%) was obtained by separation and purification by column chromatography (DCM:MeOH=80:...
Embodiment 3
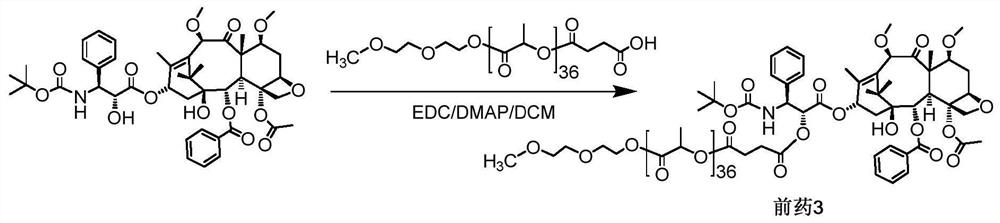
[0060] Example 3o(LA) 36 - Synthesis of CTX-coupled prodrug 3, as image 3 Shown:
[0061] Add CTX (80mg, 0.09570mmol), mPLA2600·SA (403.9mg, 0.1436mmol) and DMAP (17.5mg, 0.1436mmol) successively into a 100ml round bottom flask equipped with a spherical condenser, dissolve in 6ml of anhydrous dichloromethane , and EDC (22.3 mg, 0.1436 mmol) was added rapidly dropwise. Stir at 43°C overnight, and observe the reaction by thin-layer chromatography (developing solvent: DCM:MeOH=20:1). When the reaction was basically completed, the reaction liquid was cooled, and then washed with 5% citric acid, saturated sodium bicarbonate, and saturated saline respectively. The organic layer was dried over anhydrous sodium sulfate, and filtered after complete drying. The filtrate was rotary evaporated to remove the solvent. The final product 2 (corresponding to the structure of n=36 in formula I, 246.4 mg, yield 70.9%) was separated and purified by column chromatography (DCM:MeOH=80:1).
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com