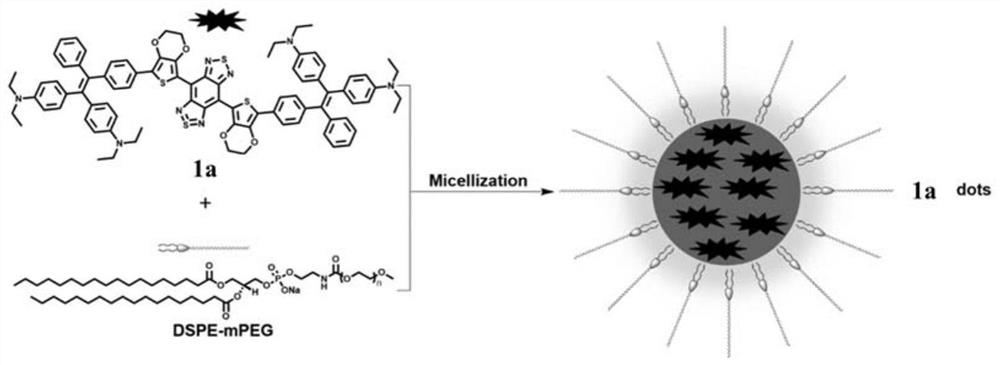
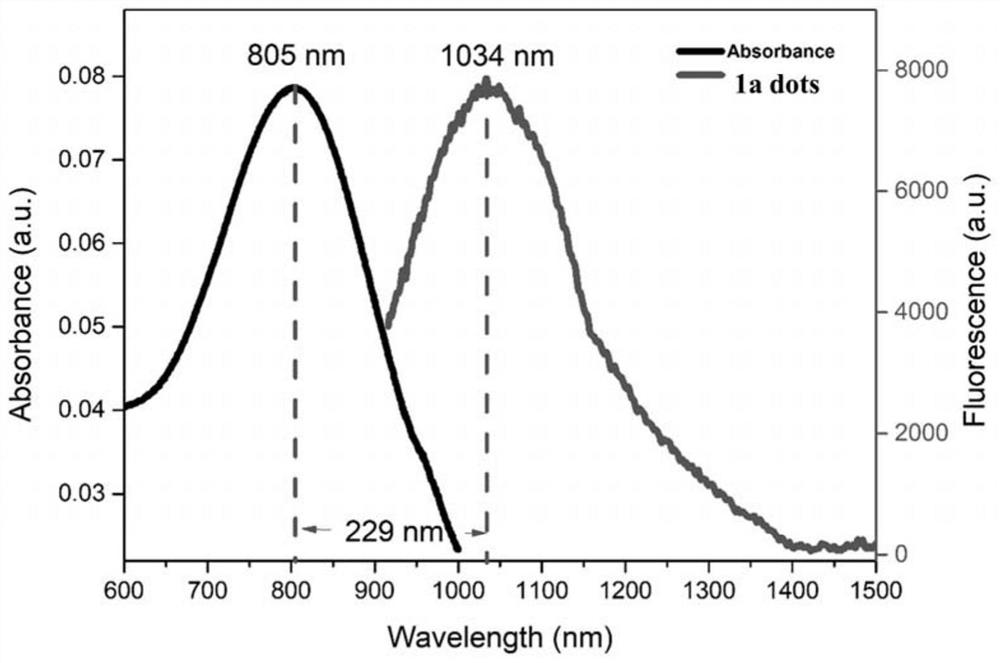
Near-infrared second-region fluorescent compound with aggregation-induced luminescent properties, preparation method, nanoparticle micelles and application thereof
A technology of aggregation-induced luminescence and fluorescent compounds, which is applied in the field of biomedical fluorescence imaging, can solve the problems of low luminous efficiency, narrowing the molecular distance, and limiting the application of dyes, etc., achieving good imaging effects, good biocompatibility, and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
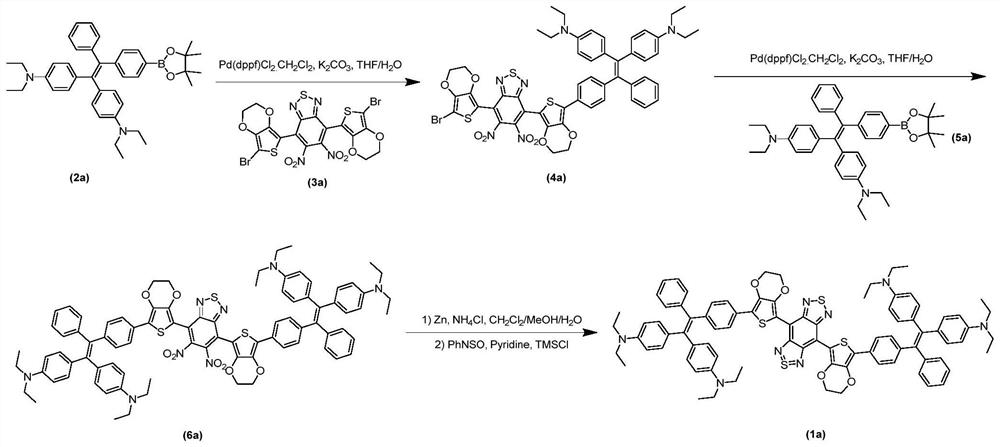
[0043] combined with Figures 1 to 10 As shown, a preparation method of the above-mentioned near-infrared two-region fluorescent compound with aggregation-induced luminescence properties includes the following route:
[0044]
[0045] The reaction conditions are:
[0046] a Under the protection of nitrogen or argon inert gas, add compound 2 and compound 3 into a reaction vessel, add tetrahydrofuran to dissolve the compounds, then pass argon or nitrogen into the reaction solution to remove oxygen in the system, add potassium carbonate aqueous solution dropwise, Weigh [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride dichloromethane complex and add it, and heat the reaction in an oil bath at 66°C-80°C under the protection of nitrogen or argon for 8 -24 hours, intermediate 4 was purified after the reaction;
[0047] b Under the protection of nitrogen or argon inert gas, take intermediate 4 and compound 5 and add them to the reaction vessel, add tetrahydrofuran to di...
Embodiment 1
[0052] Embodiment 1: the preparation of compound 4a
[0053] Take compound 2a (194mg, 0.33mmol), compound 3a (204mg, 0.31mmol) and potassium carbonate (85mg, 0.62mmol) into a 100mL round bottom flask, add tetrahydrofuran-water (v / v, 5: 1) 20mL, feed argon into the reaction solution and bubble for 5min to remove the oxygen in the system, add [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (50mg , 0.06 mmol), under the protection of argon, heated to reflux in an oil bath at 75°C for 10 hours. After the reaction, cool to room temperature, remove THF by rotary evaporation, redissolve the residue in 70 mL of dichloromethane, wash with water (40 mL×3) three times, and wash with saturated brine (40 mL×3) three times. The organic phase was dried with anhydrous magnesium sulfate for 3 hours, filtered, and the filtrate was spin-dried to obtain 334 mg of compound 4a. Yield: 75%.
[0054] The structure determination data of compound 4a are as follows:
...
Embodiment 2
[0056] Embodiment 2: the preparation of compound 6a
[0057] Take compound 4a (334mg, 0.32mmol), compound 5a (194mg, 0.33mmol) and potassium carbonate (88mg, 0.64mmol) into a 100mL round bottom flask, add tetrahydrofuran-water (v / v, 5: 1) 20mL, feed argon into the reaction solution and bubble for 5min to remove the oxygen in the system, add [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (50mg , 0.06 mmol), under the protection of argon, heated to reflux in an oil bath at 75°C for 14 hours. After the reaction, cool to room temperature, remove THF by rotary evaporation, redissolve the residue in 70 mL of dichloromethane, wash with water (40 mL×3) three times, and wash with saturated brine (40 mL×3) three times. The organic phase was dried with anhydrous magnesium sulfate for 3 hours, filtered, and the filtrate was spin-dried to obtain 345 mg of compound 6a. Yield: 72%.
[0058] The structure determination data of compound 6a are as follows:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com