Fluorene derivatives with AIE properties, preparation method and application
A technology of fluorene derivatives and aromatic hydrocarbon derivatives, which is applied in the field of aggregation-induced emission (AIE) materials, and can solve problems such as long measurement time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
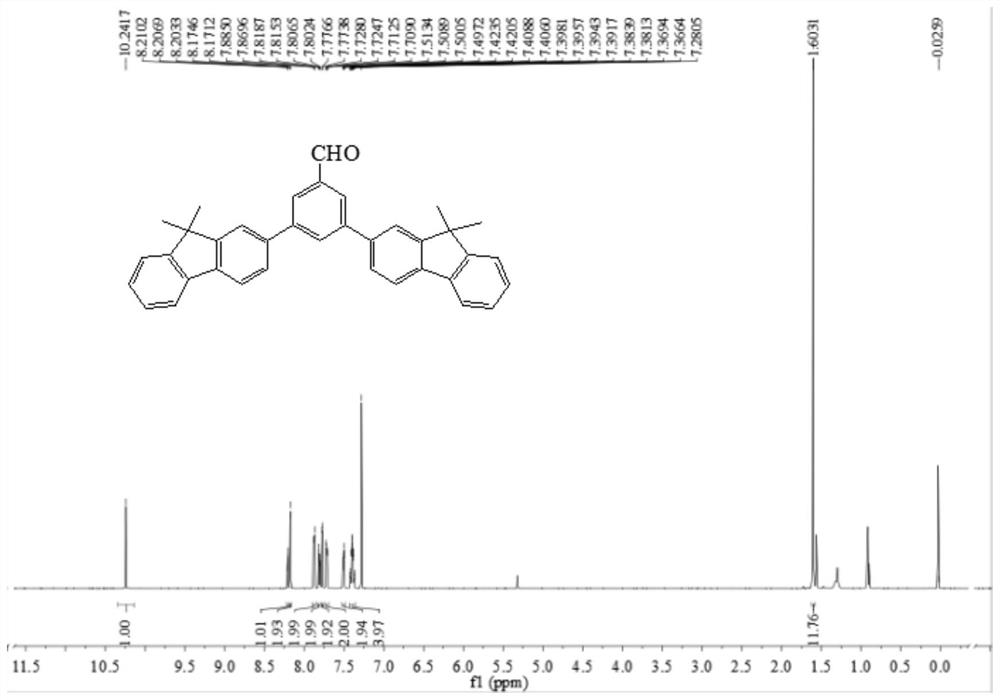
[0025] The preparation method of the fluorene derivatives with AIE characteristics of the present invention is carried out in accordance with the following steps:
[0026] Step 1: First add 5.0g fluorene and 150 mL chloroform into the bottle, then add 75 mg anhydrous ferric chloride to the bottle, stir and cool to 0°C in an ice-water bath, then add 1.6 mL liquid bromine dropwise to the bottle, pipette Remove from the ice-water bath and continue to stir for 3 hours, then add 100 mL of saturated sodium thiosulfate solution dropwise to the reaction system until the red color disappears completely, then separate the whole, extract the organic phase with dichloromethane 3-4 times, anhydrous MgSO 4 After drying, after rotary evaporation under reduced pressure, a yellow solid was obtained, which was dissolved in 80 mL of absolute ethanol, and crystals were precipitated by cooling to obtain 2-bromofluorene;
[0027] Step 2: Put 7.5 g of 2-bromofluorene, 30 mL of dimethyl sulfoxide and...
Embodiment 4
[0040] Embodiment 4: The compound is used as the competition experiment of fluorescent probe to amino acid
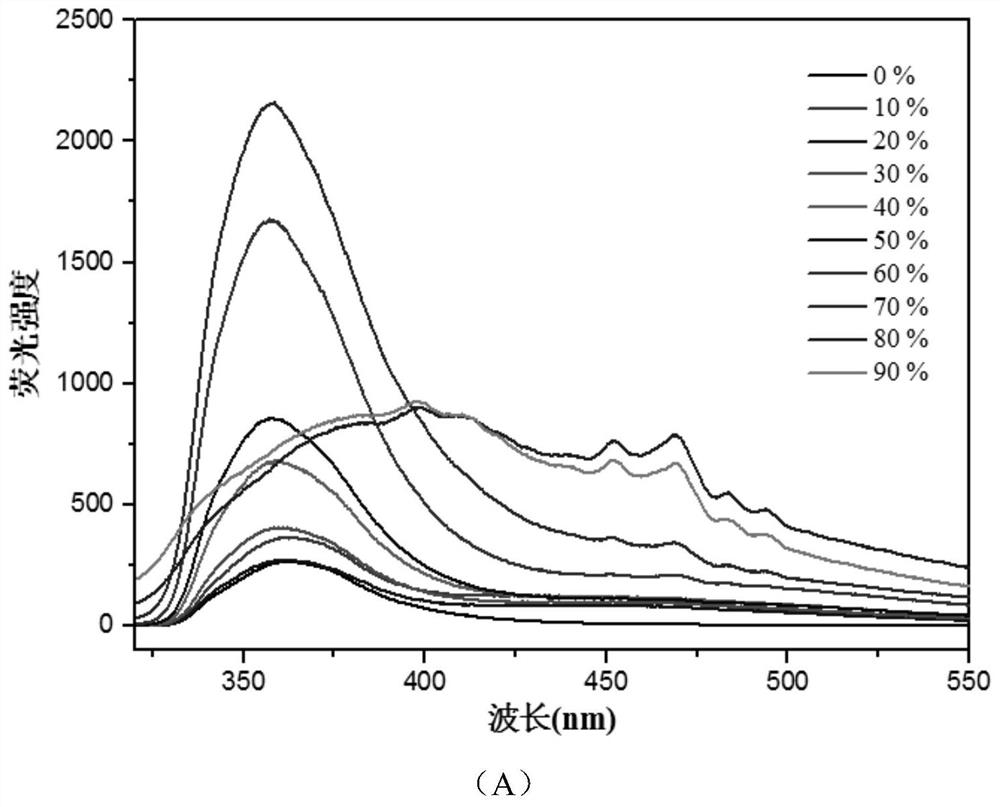
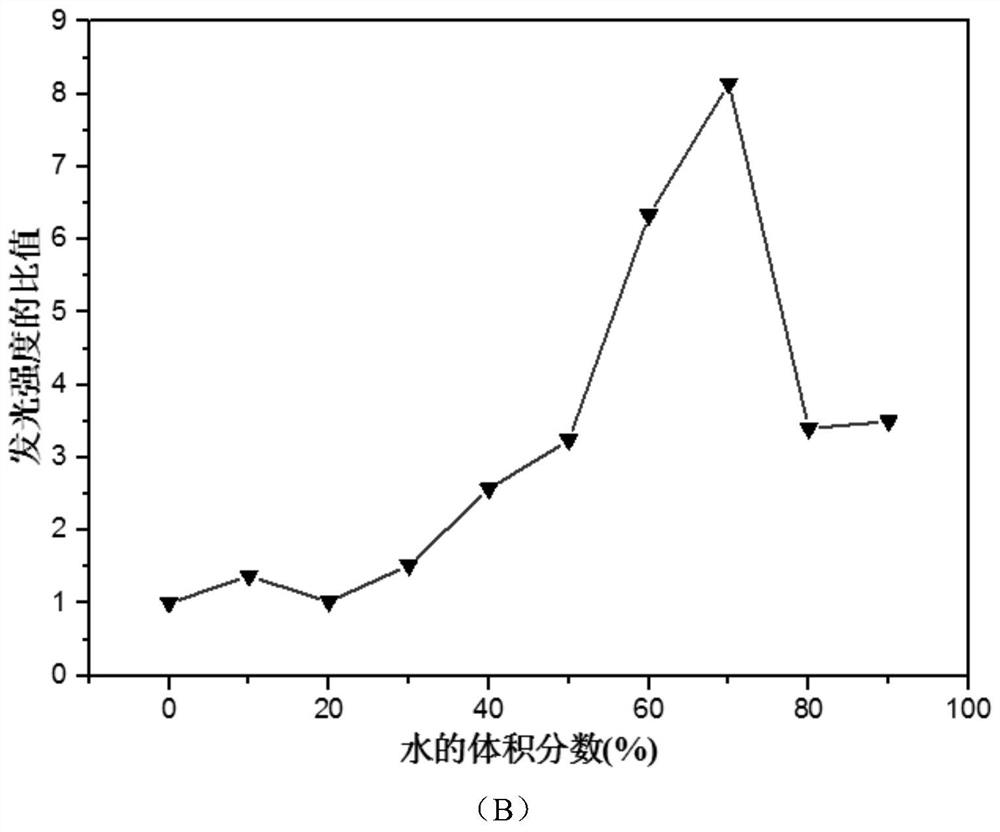
[0041] In order to determine whether the compound specifically recognizes tryptophan, leucine (Leu), serine (Ser), methionine (Met), alanine (Ala), and isoleucine (Ile) were studied respectively. , threonine (Thr), proline (Pro), arginine (Arg), lysine (Lys), aspartic acid (Asp), glutamic acid (Glu), tyrosine (Tyr) , phenylalanine (Phe), valine (Val), cysteine (Cys), histidine (His), glycine (Gly) and tryptophan (Trp) in the presence of the above amino acids Spectrum, such as Image 6 shown.
[0042] The results show that: in the presence of Trp, the emission intensity of the mixture is enhanced, while there is almost no change in the emission peak intensity in the absence of Trp, only other amino acids. It shows that even in the presence of other amino acids, the fluorescence intensity of the compound is also significantly increased under the addition of Trp, indi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com