Polymer donor material containing dibenzothiophene sulfoxide unit and preparation thereof
A technology of thiophene sulfoxide and polymers, which is applied in the field of conjugated polymer donor materials containing dibenzothiophene sulfoxide units and its preparation, can solve problems such as the performance of devices that have not been studied for blending, and achieve favorable scale Chemical production, mild reaction conditions, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
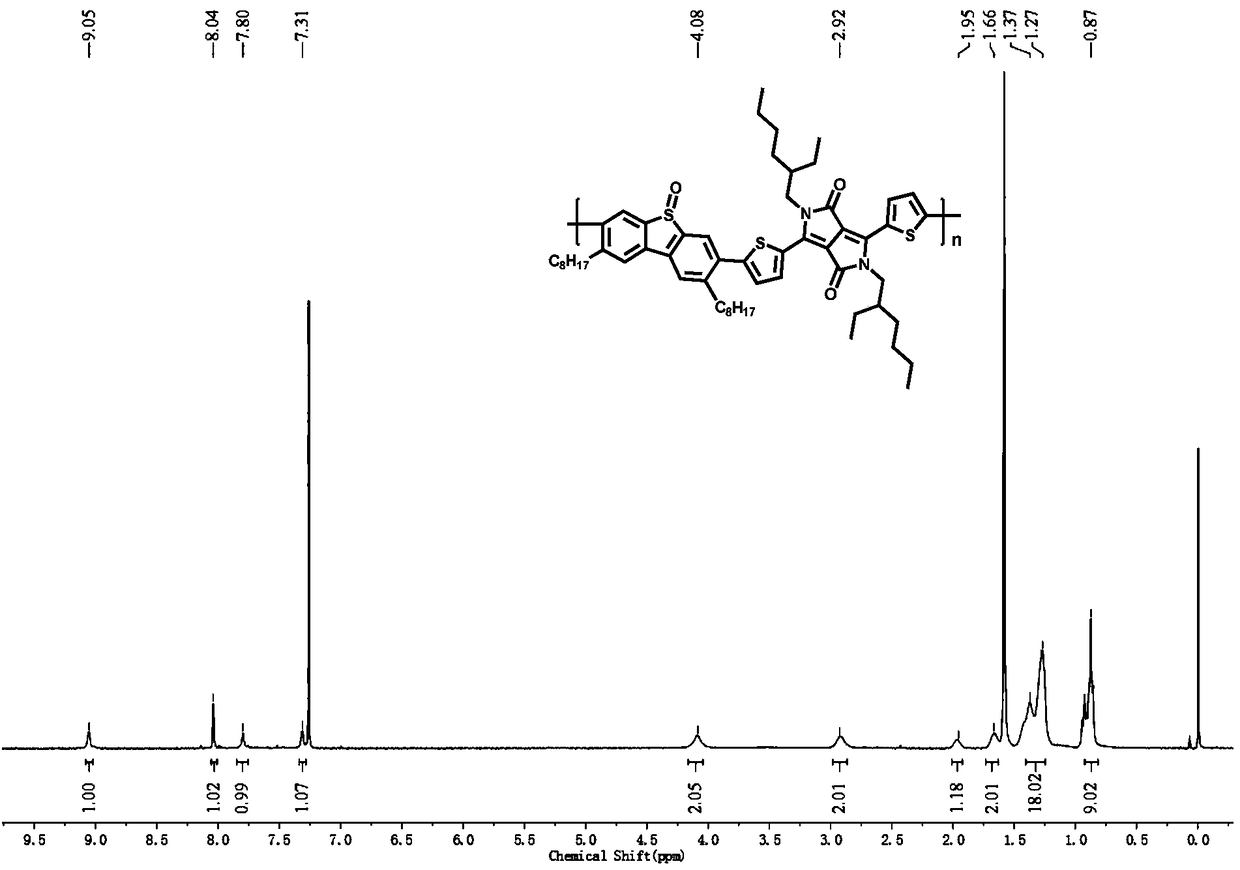
[0046] In the present embodiment, the polymer structural formula is as follows:
[0047]
[0048] The preparation of this polymer comprises the steps:
[0049] (1) Preparation of dibenzothiophene sulfoxide-based acceptor unit
[0050]
[0051] Add 2,8-dibromodibenzothiophene (5 g, 14.6 mmol), 1,3-bis(diphenyl
[0052]
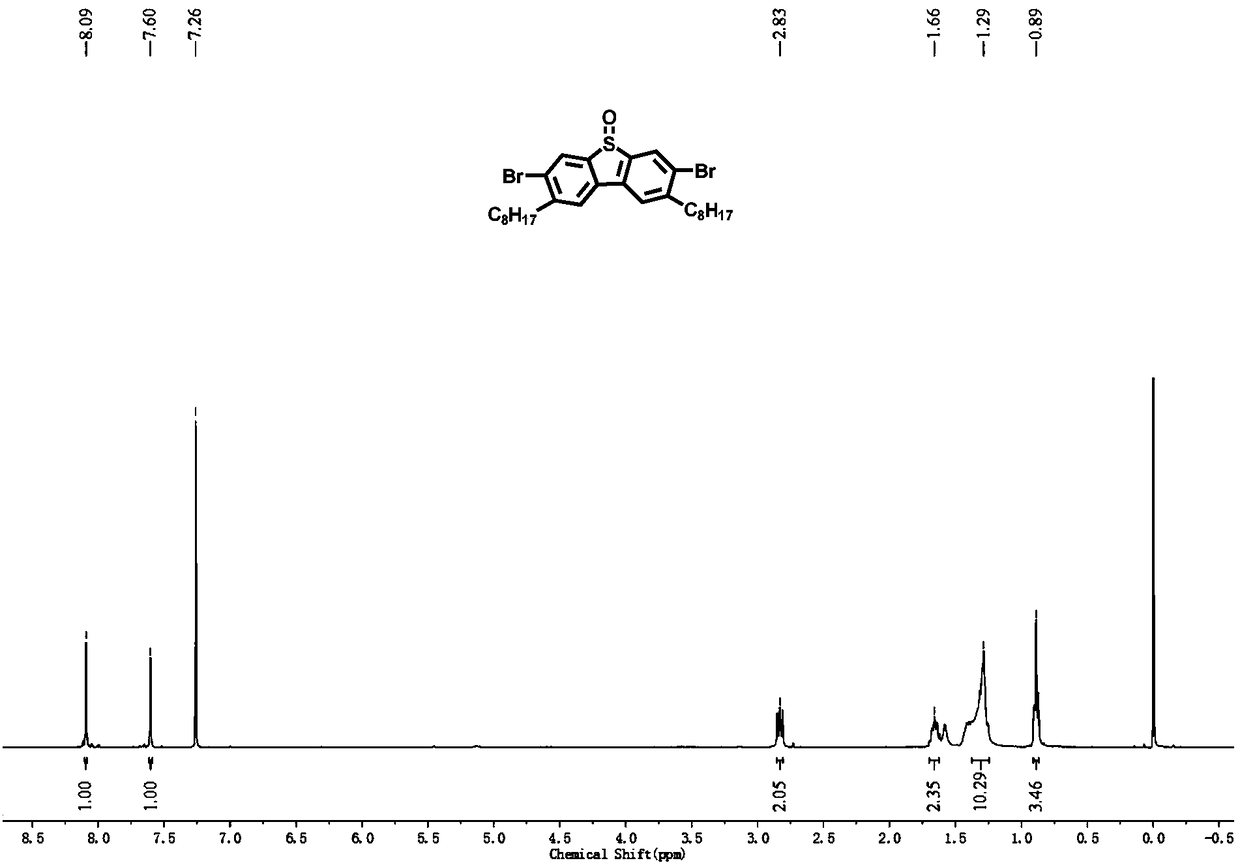
[0053] Phosphinopropane) nickel dichloride (396.2mg, 0.73mmol) and anhydrous tetrahydrofuran (200mL), under nitrogen protection, 2mol / L n-octylmagnesium bromide (29.2mL, 58.4mmol) was slowly added dropwise at 0°C, Warm up to room temperature and react for 6h. The reaction was quenched with aqueous ammonium chloride, and after rotary evaporation of most of the solution, it was extracted with dichloromethane, and the organic phases were combined and washed with anhydrous MgSO 4 dry. After the organic solvent was distilled off under reduced pressure, the residue was subjected to column chromatography using petroleum ether as the eluent to obtain a col...
Embodiment 2
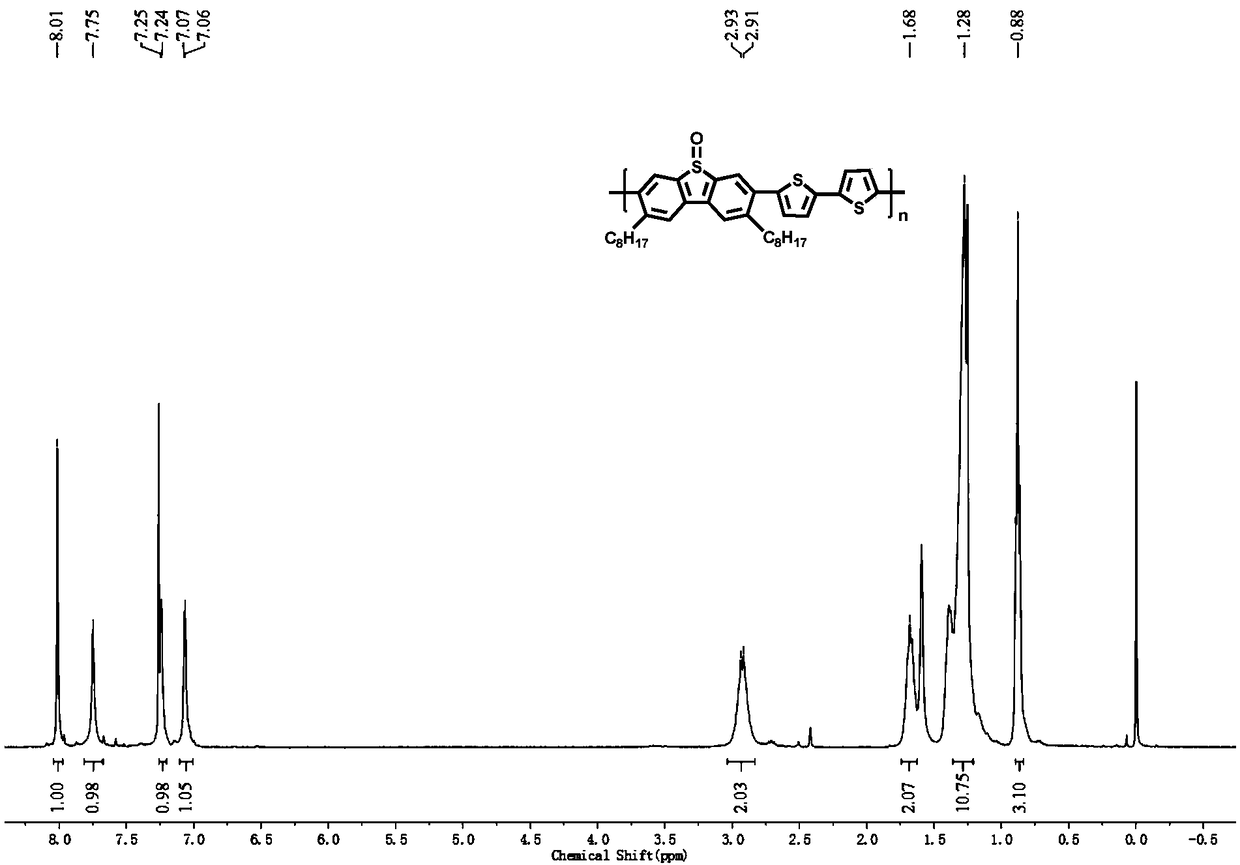
[0062] In the present embodiment, the polymer structural formula is as follows:
[0063]
[0064] The preparation of this polymer comprises the steps:
[0065]
[0066] In a 50mL single-necked flask, add 3,7-dibromo-2,8-dioctyldibenzothiophene sulfoxide (100mg, 0.17mmol), 5,5'-bistrimethyltin-2,2' -Bithiophene (84.4mg, 0.17mmol), tris(dibenzylideneacetone)dipalladium (4.8mg, 0.003mmol), tris(o-methylphenyl)phosphine (9.4mg, 0.02mmol) and toluene (6mL) , Under a nitrogen atmosphere, reflux at 110°C for 24h. After the reaction was completed, it was cooled to room temperature, and the reaction was settled in methanol. Carry out Soxhlet extraction with methanol and acetone in turn, and finally dissolve with chloroform, and dry to obtain 50 mg of orange-yellow solid, with a yield of 48%.
[0067] The proton nuclear magnetic spectrum characterization of the polymer that adopts above-mentioned method to make: 1 H NMR (400MHz, CDCl 3 )δ8.01(s,2H),7.75(s,2H),7.24(d,J=3.2Hz,2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com