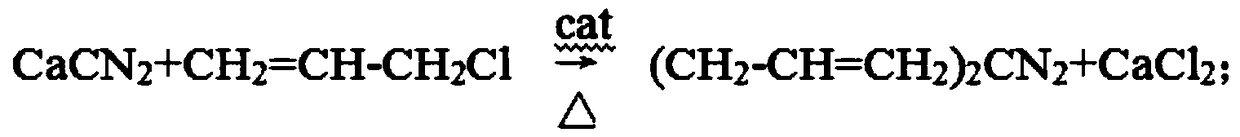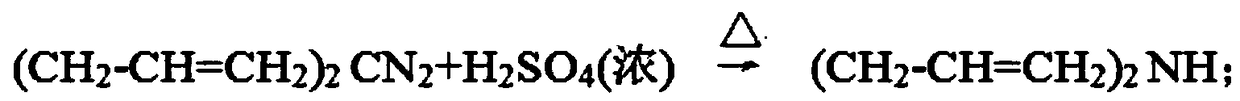Preparation method of diallylamine and hydrochloride thereof
A technology of diallylamine hydrochloride and diallylamine, which is applied in the field of preparation of diallylamine and its hydrochloride, and can solve the problems of excessive waste generation, large amount of waste water, and increased workload , to achieve the effect of less pollution and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Process for preparing diallylamine
[0049] (1) Synthesis section: pump 76.53 g of 3-chloropropene into the drip tank through a feeding pump, put 15% ammonia water into the reaction kettle, add catalyst cuprous chloride, cuprous chloride accounts for the total mass of raw materials 0.5%, the molar ratio of ammonia water to 3-chloropropene is 2.0, turn on the cooling water system to cool down to normal temperature (about 25°C), drop 3-chloropropene (completely added in 1 hour), and slowly heat up to 50- 55°C, raise the temperature by 10°C every 1 hour, control the pressure in the kettle within 0.3MPa, keep the temperature for 1 hour, then cool down to 35-40°C, after the reaction is completed, GC sampling analysis, after passing the test, enter the next section.
[0050](2) Neutralization and distillation section: open the stirring system in the neutralization distillation kettle, add quantitative 32% liquid caustic soda and recovery liquid caustic soda to the neutrali...
Embodiment 2
[0057] 1. Process for preparing diallylamine
[0058] (1) Synthesis section: 153.06 g of 3-chloropropene is pumped into the dripping tank through a feeding pump, the prepared 17% ammonia water is put into the reaction kettle, and the catalyst cuprous chloride is added, and cuprous chloride accounts for the total mass of raw materials 1%, the molar ratio of ammonia water to 3-chloropropene is 2.2:1, turn on the cooling water system to cool down to normal temperature (about 25°C), drop 3-chloropropene (completely added in 1 hour), and slowly heat up to 50-55°C, raise the temperature by 10°C every 1 hour, control the pressure in the kettle within 0.3MPa, keep the temperature for 1 hour, then cool down to 35-40°C, after the reaction is over, GC sampling analysis, after passing the test, enter the next section.
[0059] (2) Neutralization and distillation section: open the stirring system in the neutralization distillation kettle, add quantitative 32% liquid caustic soda and recove...
Embodiment 3
[0066] 1. Process for preparing diallylamine
[0067] (1) Synthesis section: 91.54g of 3-chloropropene is pumped into the dripping tank through a feeding pump, 13% ammonia water prepared is put into the reaction kettle, and the catalyst cuprous chloride is added, and the mass of cuprous chloride accounts for the total amount of raw materials. 1.5% of the mass, the molar ratio of ammonia water to 3-chloropropene is 2.5:1, turn on the cooling water system to cool down to normal temperature (about 25°C), drop 3-chloropropene (completely added in 1 hour), and slowly heat up To 50-55°C, raise the temperature by 10°C every 1 hour, control the pressure in the kettle within 0.3MPa, keep it warm for 1 hour, then lower the temperature to 35-40°C, after the reaction is completed, GC sampling analysis, after passing the test, enter the next section.
[0068] (2) Neutralization and distillation section: open the stirring system in the neutralization distillation kettle, add quantitative 32...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com