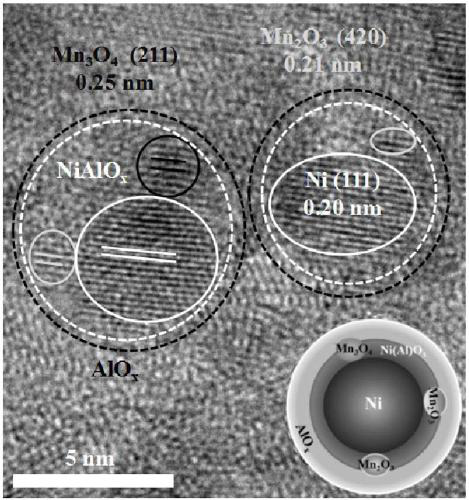
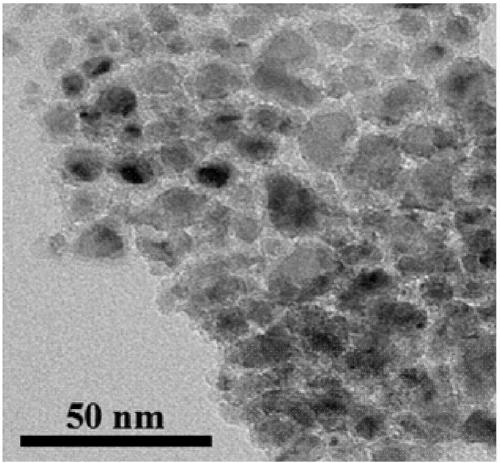
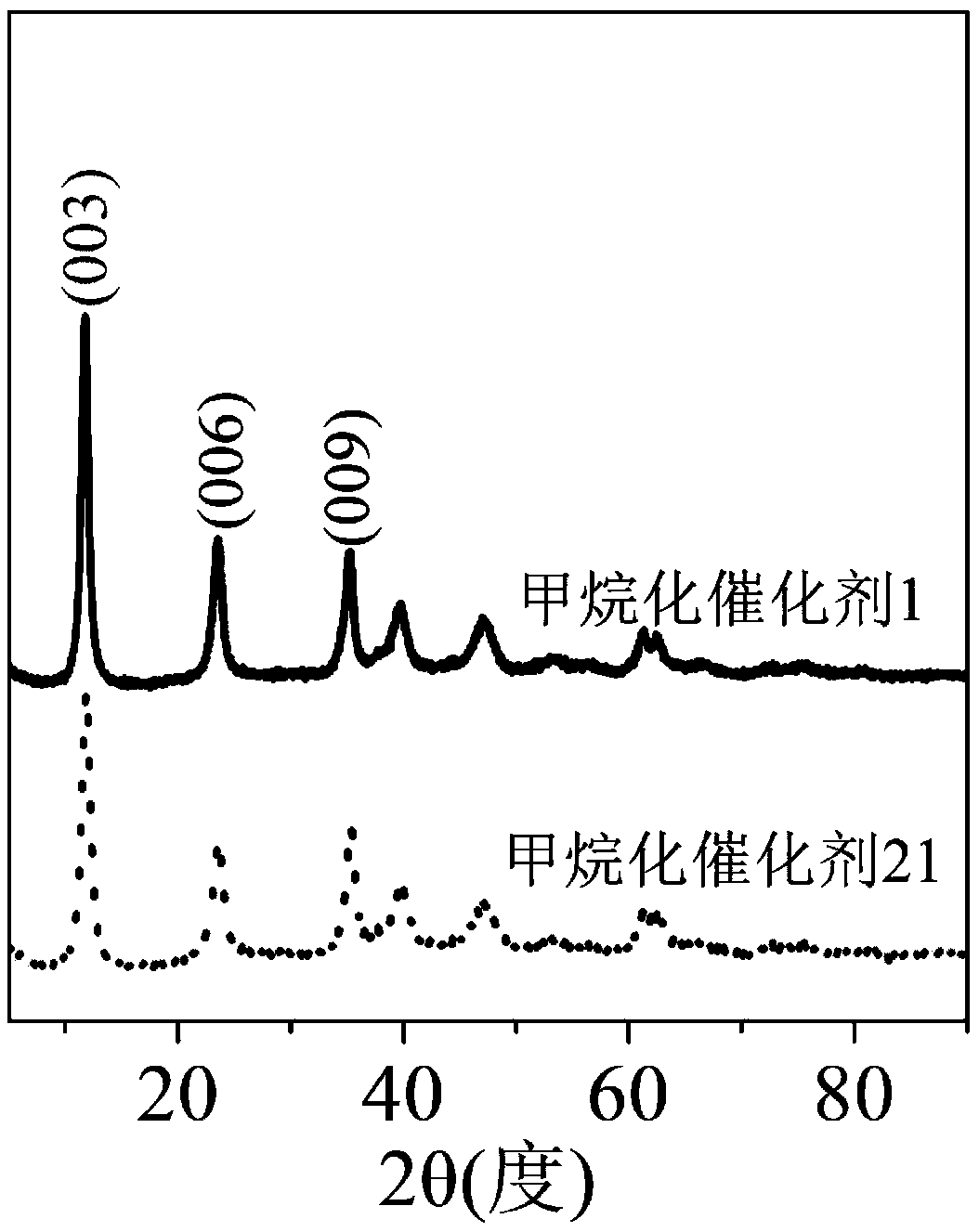
Methanation catalyst, preparation method thereof and method for using methanation catalyst to prepare methane
A methanation catalyst and lubricant technology, applied in the field of its preparation, methanation catalyst, and methane preparation, can solve problems such as difficulty in meeting actual needs, energy consumption, and environmental pollution by toxic gases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Methanation Catalyst 1 was prepared by the following method:
[0070] Step (1), 1.09g nickel nitrate (5.96mmol), 0.7g aluminum nitrate (3.28mmol) and 0.047g manganese nitrate (0.26mmol) are dissolved in 50mL deionized water, obtain nickel ion concentration and be 119mmol / L, aluminum ion The concentration of solution A is 65.6mmol / L;
[0071] Step (2), 0.6g precipitant urea (10mmol) was dissolved in 40mL deionized water to obtain solution B containing 0.25mol / L precipitant;
[0072] Step (3), mix solution A and solution B obtained in step (1) and step (2) at room temperature, place the mixed solution in a hydrothermal reaction kettle, perform hydrothermal reaction at 100°C for 30h, and the reaction ends After obtaining the crude product;
[0073] Step (4), the crude product obtained in step (3) is taken out and filtered, the filter residue is washed 2 times with deionized water, after drying at 120° C. for 20 h, the lubricant graphite and The weight percentage is the fo...
Embodiment 2
[0075] Methanation Catalyst 2 was prepared by the following method:
[0076] The only difference from Example 1 is that the same molar amount of indium nitrate is used in step (1) instead of manganese nitrate to add to solution A.
[0077] Embodiment 2 obtains methanation catalyst 2.
Embodiment 3
[0079] Methanation catalyst 3 was prepared by the following method:
[0080] The only difference from Example 1 is that in step (1), the same molar amount of nickel acetate and aluminum acetate is used instead of nickel nitrate and aluminum nitrate, and the same molar amount of ammonium molybdate is used instead of manganese nitrate to add to solution A.
[0081] Example 3 to obtain the methanation catalyst 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com