Creatininase method detection kit
A detection kit and creatinase technology, applied in the field of medical testing, can solve problems such as environmental pollution and high toxicity, and achieve the effects of strong anti-interference ability, high accuracy and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
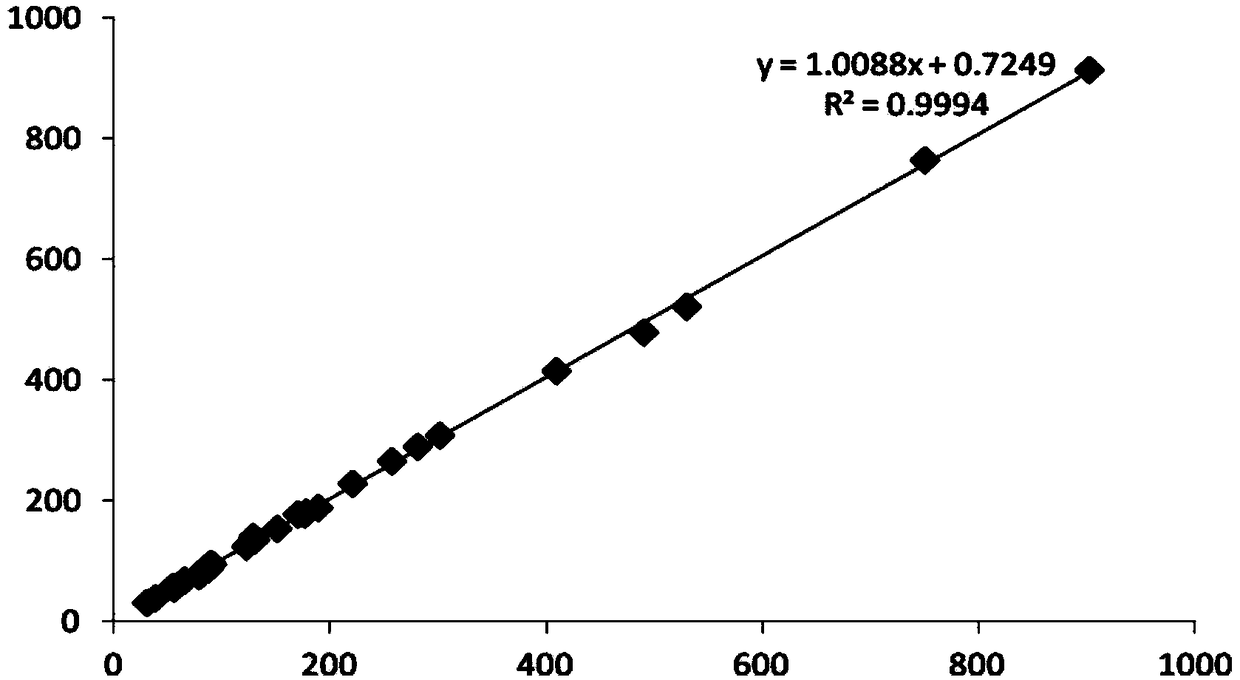
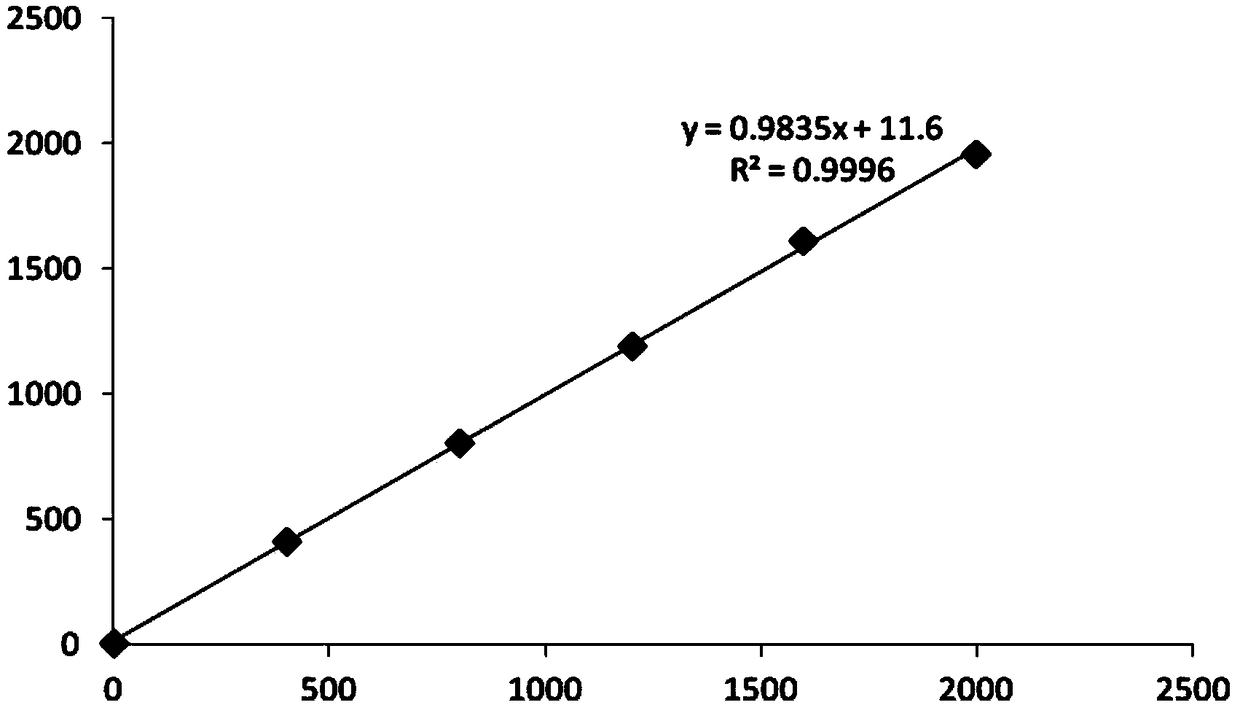
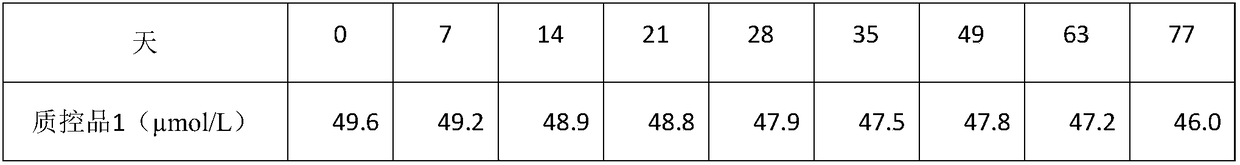
Image
Examples
Embodiment 1
[0045] The creatinine enzymatic detection kit of this embodiment is composed of reagent R1 and reagent R2, wherein:
[0046] The composition of reagent R1 is: Tris buffer: 20mmol / L, pH7.50; creatinase: 20KU / L; sarcosine oxidase: 15.2KU / L; ascorbate oxidase: 18KU / L; bilirubin oxidase: 80KU / L; TOOS 2mmol / L; peroxidase 40KU / L; BSA 20g / L; trehalose 10g / L; Tween20 0.3g / L; TgHb 1-5 0.5g / L.
[0047] The composition of reagent R2 is: Tris buffer: 30mmol / L, pH7.80; 4-aminoantipyrine 3mmol / L; creatinase 400KU / L; BSA 5g / L; Tween80 0.3g / L; TgHb 1-5 0.4 g / L.
Embodiment 2
[0049] The creatinine enzymatic detection kit of this embodiment is composed of reagent R1 and reagent R2, wherein:
[0050] The composition of reagent R1 is: HEPES buffer: 50mmol / L, pH8.0; creatinase: 40KU / L; sarcosine oxidase: 29KU / L; ascorbate oxidase: 50KU / L; bilirubin oxidase: 40KU / L; TOOS 5mmol / L; peroxidase 60KU / L; sucrose 10g / L; TritonX-100 0.2g / L; Aurelin 0.2g / L.
[0051] The composition of reagent R2 is: PIPES buffer: 50mmol / L, pH8.2; 4-aminoantipyrine 5mmol / L; creatinase 600KU / L; fructose 15g / L; TritonX-100 0.5g / L; Aurelin 0.8g / L.
Embodiment 3
[0053] The creatinine enzymatic detection kit of this embodiment is composed of reagent R1 and reagent R2, wherein:
[0054] The composition of reagent R1 is: MOPS buffer: 30mmol / L, pH8.5; creatinase: 62KU / L; sarcosine oxidase: 35KU / L; ascorbate oxidase: 80KU / L; bilirubin oxidase: 15KU / L; TOOS 6mmol / L; peroxidase 45KU / L; glycerol 20g / L; SPAN20 0.1g / L; MCdef: 0.6g / L.
[0055] Reagent R2 consists of: MPOS buffer: 50mmol / L, pH8.4; 4-aminoantipyrine 6mmol / L; creatinase: 700KU / L; mannitol: 10g / L; glycerol: 10g / l; SPAN20 0.21 g / L; MCdef: 0.4g / L.
[0056] In the present invention, TgHb 1-5 is clam antimicrobial peptide (shellfish), prepared according to Chinese patent application CN2017104041998; Aurelin is jellyfish antimicrobial peptide, prepared according to Chinese patent application CN2017104041983; MCdef is clam antimicrobial peptide, obtained according to Chinese patent application CN2017104041983 Patent application CN2017104041964 is prepared; or in the present invention, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com