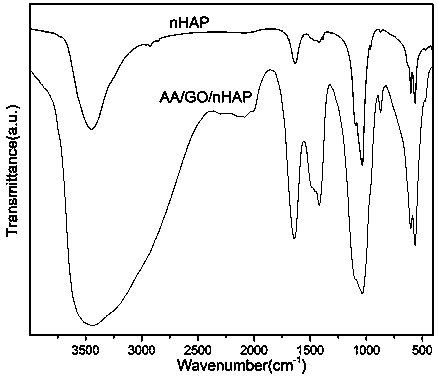
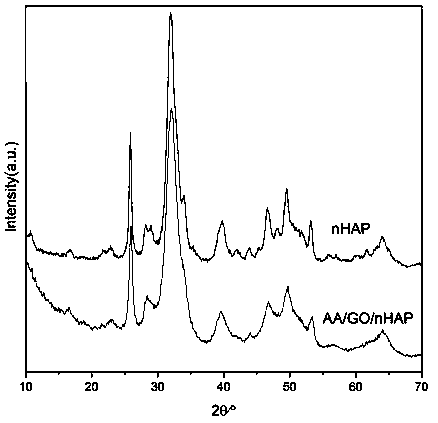
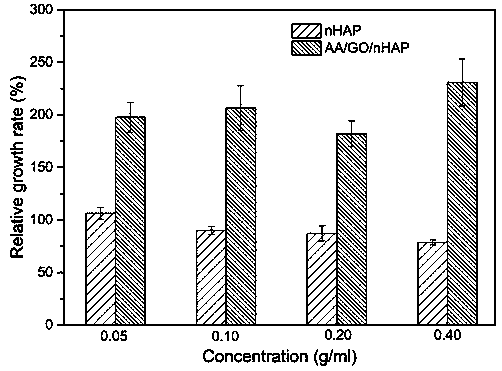
Preparation method of dental restoration modified nano-hydroxyapatite composite material
A nano-hydroxyapatite, composite material technology, applied in dental preparations, phosphorus compounds, nanotechnology and other directions, can solve the problems of unfavorable anti-caries repair, poor antibacterial performance of nHAP, high crystallinity and structural stability, and achieve biological Easy retention of activity, in situ repair, good antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Weigh 3.542g of calcium nitrate tetrahydrate and dissolve it in 400ml of deionized water. After fully dissolving, add 1.126g of glycine and 1.997g of aspartic acid respectively to make solution A for later use; the molar ratio of soluble calcium salt to compound amino acid It is 1:2.
[0026] (2) Weigh 1.186g of diammonium hydrogen phosphate and dissolve it in 200ml of deionized water, add 0.0593g of graphene oxide powder, ultrasonically disperse evenly, and prepare solution B for later use, in which the mass ratio of soluble phosphate to graphene oxide is 1: 0.05.
[0027] (3) Prepare a pH regulator with a sodium hydroxide concentration of 0.02mol / L for use.
[0028] (4) Add solution A and solution B into a 1L autoclave in sequence under stirring (the stoichiometric ratio of Ca / P in soluble calcium salt and soluble phosphate is 1.67), and adjust the pH value of the reaction solution to 9.0~10.0.
[0029] (5) After hydrothermal reaction in a high-pressure reactor...
Embodiment 2
[0032] (1) Weigh 3.542g of calcium nitrate tetrahydrate and dissolve it in 400ml of deionized water. After fully dissolving, add 0.105g of serine and 0.076g of glutamic acid respectively to prepare solution A for use. The molar ratio of soluble calcium salt to compound amino acid is 1:0.1.
[0033] (2) Weigh 1.186g of diammonium hydrogen phosphate and dissolve it in 200ml of deionized water to make solution B for later use.
[0034] (3) Prepare a pH regulator with a sodium hydroxide concentration of 0.02mol / L for use.
[0035] (4) Add solution A and solution B into a 1L autoclave in sequence under stirring (the stoichiometric ratio of Ca / P in soluble calcium salt and soluble phosphate is 1.67), and adjust the pH value of the reaction solution to 10.0~11.0.
[0036] (5) After hydrothermal reaction in a high-pressure reactor at 120°C for 12 hours, cool down, wash the reaction suspension until the pH of the supernatant is neutral, filter and freeze-dry it to prepare amino acid-...
Embodiment 3
[0039] (1) Weigh 3.542g of calcium nitrate tetrahydrate and dissolve it in 400ml of deionized water. After fully dissolving, add 3.153g of serine, 4.414g of glutamic acid and 3.994g of aspartic acid to make solution A for later use. The soluble calcium salt The molar ratio with compound amino acid is 1:6.
[0040] (2) Weigh 1.186g of diammonium hydrogen phosphate and dissolve it in 200ml of deionized water, add 0.1186g of graphene oxide powder, ultrasonically disperse evenly, and prepare solution B for later use, in which the mass ratio of soluble phosphate to graphene oxide is 1: 0.1.
[0041] (3) Prepare a pH regulator with a sodium hydroxide concentration of 0.02mol / L for use.
[0042] (4) Add solution A and solution B into a 1L autoclave in sequence under stirring (the stoichiometric ratio of Ca / P in soluble calcium salt and soluble phosphate is 1.67), and adjust the pH value of the reaction solution to 8.0~9.0.
[0043] (5) After hydrothermal reaction in an autoclave a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com