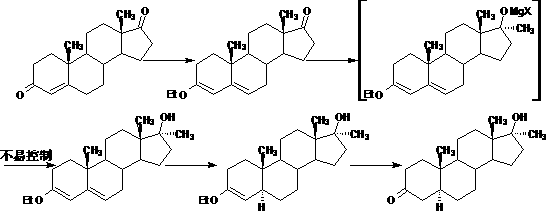
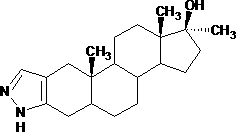
Method for synthesizing stanozolol intermediate androstane-17alpha-methyl-17beta-hydroxyl-3-ketone
A technology for the synthesis of stanozolol and its synthesis method, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of similar product polarity, influence on product purity, difficulty in removal, etc., and achieve the advantages of short route, easy control of production process and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
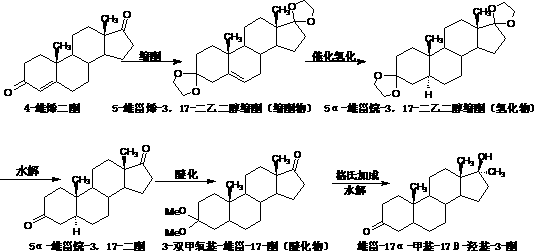
[0034] 1. Preparation of compound 5-androstene-3,17-diethylene glycol ketal
[0035] Add 20g of 4-androstenedione, 100ml of dichloromethane, 30ml of trimethyl orthoformate, 40ml of ethylene glycol and 1g of p-toluenesulfonic acid into the reaction bottle, stir well and keep warm at 30°C to 35°C for 10 hours to react; After the reaction is complete, lower the temperature to below 5°C, add triethylamine dropwise to adjust the pH value to 7.0-7.5, and stir for 30 minutes after addition; evaporate all solvents under reduced pressure, entrain with 20ml of methanol, and concentrate under reduced pressure to a paste. Add 20ml of methanol and reflux for 30 minutes; cool down to below 5°C, filter and dry to obtain 24.8g of 5-androstene-3,17-diethylene glycol ketal;
[0036] 2. Preparation of compound 5α-androstane-3,17-dione
[0037] Add 20g of 5-androstene-3,17-diethylene glycol ketal and 400ml of ethanol to the reaction flask, stir well, add potassium hydroxide ethanol solution drop...
Embodiment 2
[0045] 1. Preparation of compound 5-androstene-3,17-diethylene glycol ketal
[0046] Add 20g of 4-androstenedione, 80ml of chloroform, 40ml of triethyl orthoformate, 60ml of ethylene glycol and 2g of pyridine hydrochloride into the reaction flask, stir evenly and keep warm at 30°C to 35°C for 9 hours to react; After completion, lower the temperature to below 5°C, add triethylamine dropwise to adjust the pH value to 7.0-7.5, and stir for 30 minutes after addition; evaporate all solvents under reduced pressure, entrain with 20ml methanol, concentrate under reduced pressure to paste, add 20ml Methanol was refluxed for 30 minutes; the temperature was lowered to below 5°C, filtered and dried to obtain 24.2g of 5-androstene-3,17-diethylene glycol ketal;
[0047] 2. Preparation of compound 5α-androstane-3,17-dione
[0048] Add 20g of 5-androstene-3,17-diethylene glycol ketal and 300ml of methanol into the reaction flask, stir evenly, add methanol solution of sodium hydroxide dropwis...
Embodiment 3
[0056] 1. Preparation of compound 5-androstene-3,17-diethylene glycol ketal
[0057] Add 20g of 4-androstenedione, 120ml of dichloromethane, 60ml of triethyl orthoformate, 60ml of ethylene glycol and 1g of p-toluenesulfonic acid into the reaction flask, stir evenly and keep warm at 30°C to 35°C for 9 hours for reaction; After the reaction is complete, lower the temperature to below 5°C, add triethylamine dropwise to adjust the pH value to 7.0-7.5, and stir for 30 minutes after addition; evaporate all solvents under reduced pressure, entrain with 20ml of methanol, and concentrate under reduced pressure to a paste. Add 20ml of methanol and reflux for 30 minutes; cool down to below 5°C, filter and dry to obtain 25.1g of 5-androstene-3,17-diethylene glycol ketal;
[0058] 2. Preparation of compound 5α-androstane-3,17-dione
[0059] Add 20g of 5-androstene-3,17-diethylene glycol ketal and 300ml of methanol into the reaction flask, stir evenly, add potassium hydroxide ethanol solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com