A method for synthesizing and purifying Tyramycin impurity C
A technology of telamycin and impurity, which is applied in the field of synthesis and purification of telamycin impurity C, can solve the problems of low purity of telamycin impurity C, unsatisfactory structure and properties, and difficult control of the synthesis process, so as to achieve complete purification , stable properties and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
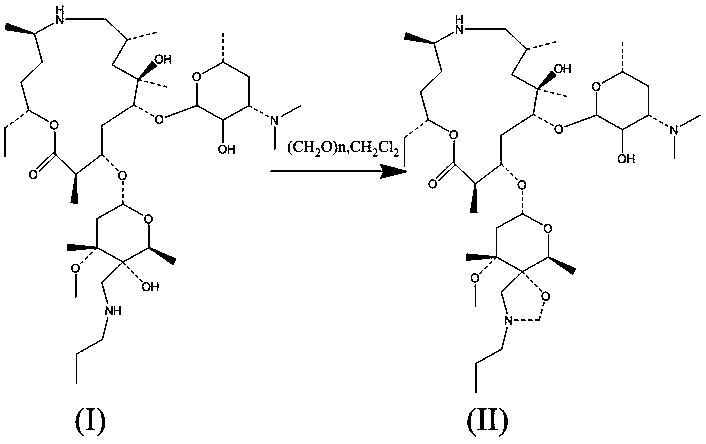
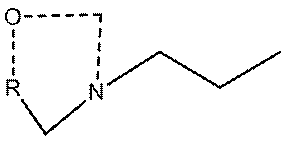
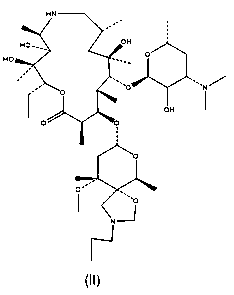
[0031] A. Put 20g of dry powder of Tyramycin into the container, add 200g of dichloromethane to dissolve, control the temperature at 30°C, add 2.0g of paraformaldehyde, 0.1g of tetrabutylammonium bromide, into a 500ml four-necked flask, and stir for 36 hours. ;
[0032] B, sampling detection, the residual amount of Tyramycin is 15%, continue to react for 14 hours; Sampling detection again, the residual amount of Tyramycin is 6.5%, further crystallize the reaction solution;
[0033] C. During crystallization treatment, add 200ml of water to the reaction solution, stir for 30 minutes, and separate layers; the dichloromethane layer is concentrated to dryness, add 50g of methanol to dissolve, add 100g of purified water dropwise, crystallize at 0°C for 2 hours, and extract the white crystals Filter and dry to obtain the crude product of Tyramycin impurity C;
[0034] D. Recrystallize the obtained Tyramycin impurity C crude product, add 30 g of acetone to dissolve 15 g of Tyramycin...
Embodiment 2
[0036] A. Put 20g of dry powder of Tyramycin into the container, add 200g of dichloromethane to dissolve, control the temperature at 30°C, add 5.0ml of formaldehyde, 1.5g of tetrabutylammonium bromide, into a 500ml four-necked flask, and stir for 24 hours;
[0037] B, sampling detection, the residual amount of Tyramycin is 14.5%, continue to react for 13 hours; Sampling and detection again, the residual amount of Tyramycin is 7%, and further crystallization is carried out to the reaction solution;
[0038] C. During crystallization, add 200ml of water to the reaction solution, stir for 30 minutes, and separate layers; the dichloromethane layer is concentrated and dried, add 50g of acetone to dissolve, add 100g of purified water dropwise, crystallize at 4°C for 2 hours, and filter the white crystals with suction , dried to obtain the crude product of Tyramycin impurity C;
[0039] D. Recrystallize the obtained Tyramycin impurity C crude product, add 30 g of acetone to dissolve ...
Embodiment 3
[0041] A. Put 20 g of dry powder of Tyramycin into the container, add 200 g of dichloromethane to dissolve, control the temperature at 40°C, put 2.0 g of paraformaldehyde, 2.0 g of tetrabutylammonium bromide in a 500 ml four-necked flask, and stir for 28 hours;
[0042] B, sampling detection, the residual amount of Tyramycin is 12.7%, continue to react for 12 hours; Sampling detection again, the residual amount of Tyramycin is 7.2%, and further crystallization is carried out to the reaction solution;
[0043] C. During the crystallization treatment, add 200ml of water to the reaction solution, stir for 30 minutes, and separate layers; Suction filtration of crystallization and drying to obtain the crude product of impurity C of Tyramycin;
[0044] D, recrystallize the obtained Tyramycin impurity C crude product, add 30g dichloromethane to dissolve 15g of Tyramycin C crude product, add dropwise 60g n-heptane, after the dropwise addition, white crystals are separated out, crystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com