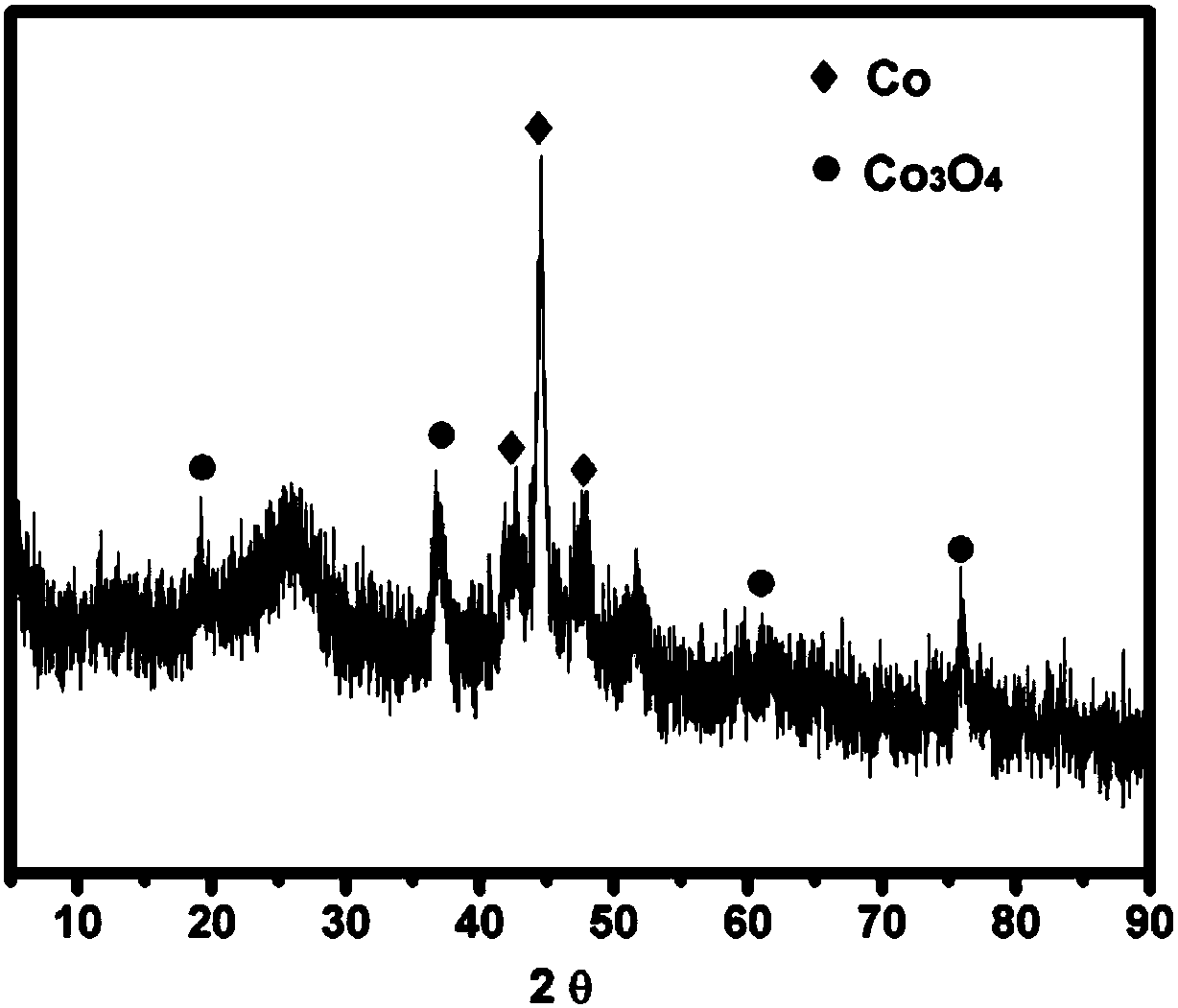
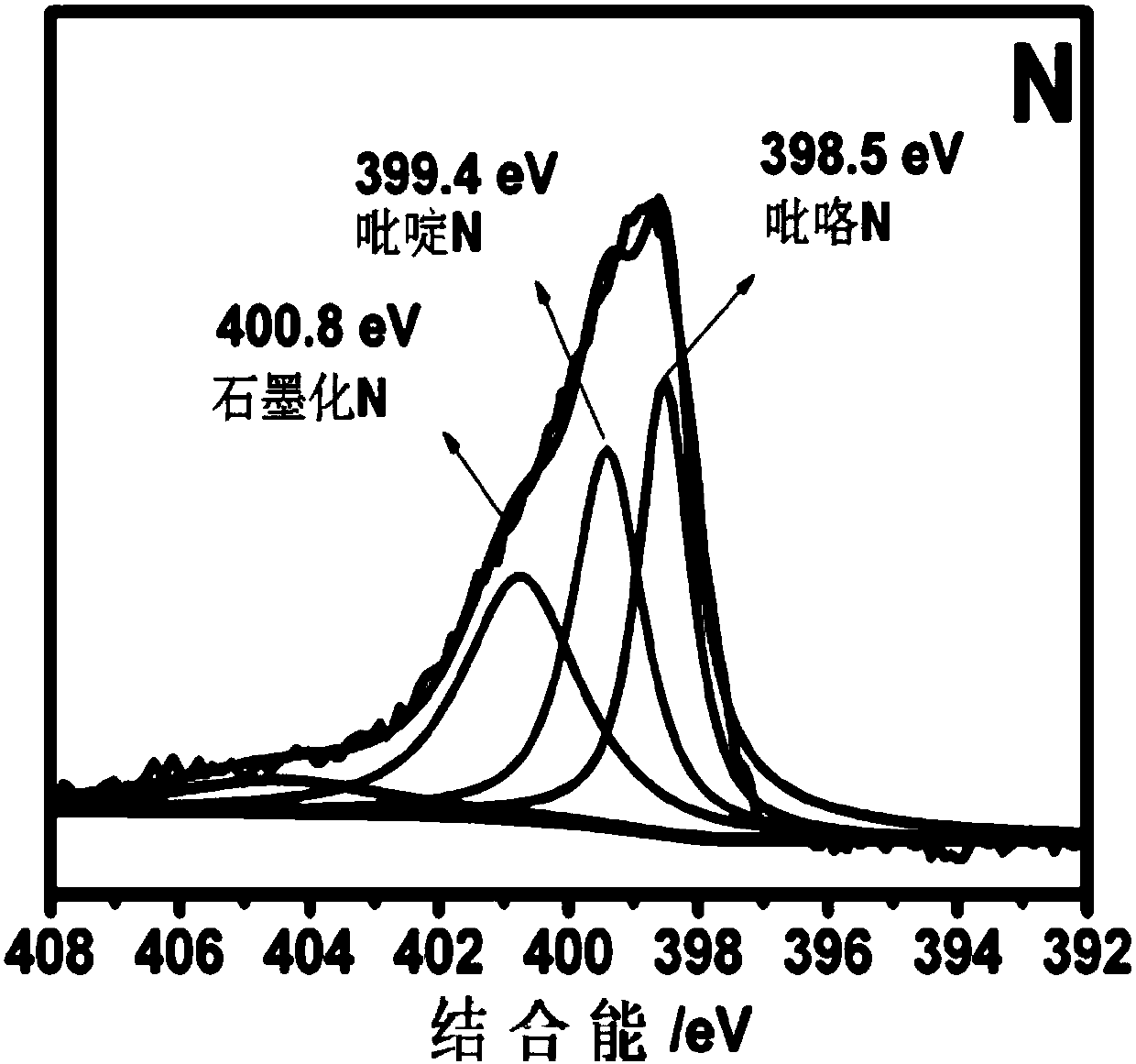
Heteroatom-doped carbon-based non-noble metal compound electrocatalyst and preparation method thereof
A non-precious metal and compound electrotechnology, applied in the field of electrocatalysis, can solve the problems of many preparation steps, low degree of bonding, high cost of raw materials or equipment, etc., achieve high nitrogen doping, promote complete decomposition, and reduce stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Phosphor flake graphite is prepared by the Hummers oxidation method to obtain graphene oxide, and the graphene oxide aqueous solution is obtained by dialysis. Measure the graphene oxide aqueous solution containing 100 mg of graphene oxide and dilute to 100 mL with deionized water.
[0069] Sinter melamine at 550° C. in air atmosphere to obtain graphitic carbon nitride raw material, sonicate 100 mg of graphitic carbon nitride in hydrochloric acid (37%) for 3 hours, filter and wash to obtain acidified graphitic carbon nitride.
[0070] The above-mentioned acidified graphite phase carbon nitride and graphene oxide aqueous solution were mixed, ultrasonically treated for 0.5 hours, and 200mg Co(NO 3 ) 2 ·6H 2 O and stirred for 0.5 hours, added 10mL of ammonia water, stopped stirring, filtered, and washed to obtain a composite precursor: cobalt hydroxide / acidified graphite phase carbon nitride / graphene oxide. Finally, the composite precursor was treated in a nitrogen atmos...
Embodiment 2
[0074] Phosphor flake graphite is prepared by the Hummers oxidation method to obtain graphene oxide, and the graphene oxide aqueous solution is obtained by dialysis. Measure the graphene oxide aqueous solution containing 100 mg of graphene oxide and dilute to 100 mL with deionized water.
[0075] Dicyandiamide was sintered at 550°C in air atmosphere to obtain graphitic carbon nitride raw material, 100 mg of graphitic carbon nitride was ultrasonically treated in hydrochloric acid (37%) for 3 hours, filtered and washed to obtain acidified graphitic carbon nitride.
[0076] The above-mentioned acidified graphite phase carbon nitride and graphene oxide aqueous solution were mixed, ultrasonically treated for 0.5 hours, and 200mg Co(NO 3 ) 2 ·6H 2 O and stirred for 0.5 h, adding 500 mg Na 2 S·9H 2 O and continue to stir for 0.5 hours, filter and wash to obtain a composite precursor: cobalt sulfide / acidified graphite phase carbon nitride / graphene oxide. Finally, the composite pr...
Embodiment 3
[0080] 100 mg of carbon nanotubes were treated at 80° C. for 6 hours with a mixture of concentrated nitric acid and concentrated sulfuric acid at a volume ratio of 1:3. The obtained modified carbon nanotubes (with oxygen-containing groups on the surface) were dispersed in 100 mL of deionized water.
[0081] Sinter urea at 550° C. in air atmosphere to obtain graphitic carbon nitride raw material, ultrasonically treat 50 mg of graphitic carbon nitride in hydrochloric acid (37%) for 3 hours, filter and wash to obtain acidified graphitic carbon nitride.
[0082] The above-mentioned acidified graphite phase carbon nitride and the aqueous solution of modified carbon nanotubes were mixed, ultrasonically treated for 0.5 hours, and 200mg Co(NO 3 ) 2 ·6H2 O and stirred for 0.5 h, adding 500 mg Na 2 S·9H 2 O and continue to stir for 0.5 hours, filter and wash to obtain the composite precursor: cobalt sulfide / acidified graphite phase carbon nitride / modified carbon nanotubes. Finally, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com