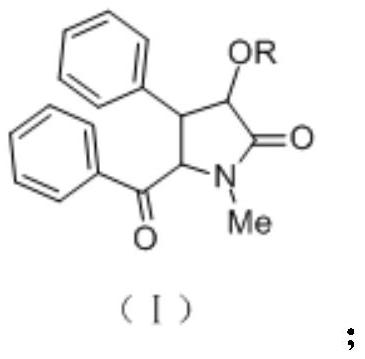
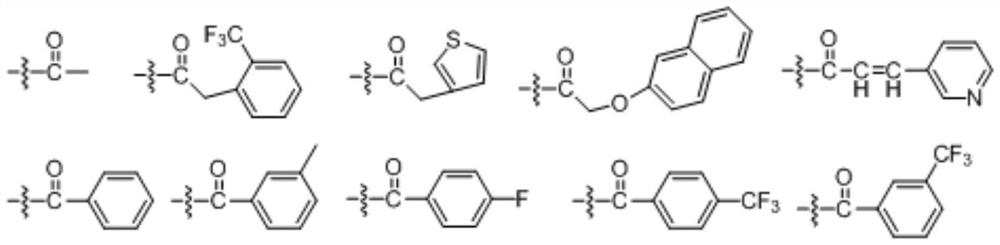
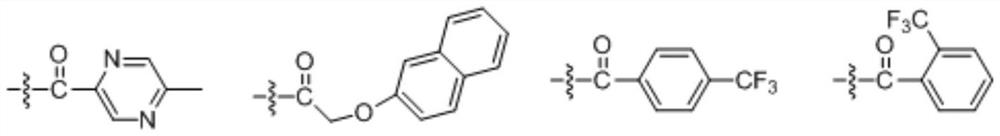
A kind of optically active xanthamide ketone derivative and its application
A kind of xanthinone and optically active technology, which is applied in the field of biomedicine, can solve the problems of further research and development, untargeted research results on the structure or type of symptomatic compounds, etc., so as to reduce nerve cell damage and have a novel structure , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The preparation of embodiment 1 (-)-bantamide ketone or (+)-bantamide ketone
[0078] The preparation process of (-)-bantamide ketone or (+)-bantamide ketone is as follows:
[0079] 1. Synthesis and resolution of racemic epoxy cinnamic acid ((±)-β-phenylglycidyl acid):
[0080]
[0081] ①Synthesis of racemic epoxy cinnamic acid ((±)-β-phenylglycidyl acid)
[0082]Weigh 148.20g of trans-cinnamic acid, dissolve it in 650mL of acetone, add 380.0g of sodium bicarbonate under stirring, cool to 0-20°C in an ice bath, add 650mL of water, then add 620.0g of potassium persulfate in 1250mL of aqueous solution, keep The temperature is lower than 20°C. After reacting for 3 hours, filter out insoluble salts, add 1.0L ethyl acetate to the filtrate and stir, adjust the pH to 5-6 with concentrated hydrochloric acid, separate the organic layer, and extract the aqueous layer with 3×500mL ethyl acetate , the organic layers were combined, washed with 1L of distilled water and 1L of sa...
Embodiment 2
[0097] The preparation of embodiment 2 compound (+)-58 and (-)-58
[0098] 1. Preparation of (-)-58:
[0099]
[0100] Weigh 0.590g (2mmol) (-)-Panthamide ketone, 1.150g (6mmol) DCC (dicyclohexylcarbodiimide) and 0.049g (0.4mmol) DMAP (4-dimethylaminopyridine) dissolved in 50mL Dichloromethane, add 6mmol p-trifluoromethylbenzoic acid under stirring at room temperature, TLC monitoring (EA:PE=2:1), after the reaction is complete, successively add 30mL 1M HCl, 30mL saturated sodium bicarbonate and 30mL saturated sodium chloride The solution was washed, dried over anhydrous sodium sulfate, the desiccant was filtered off, and the solvent was spinned off to obtain a crude product, which was subjected to column chromatography and recrystallized from methanol to obtain compound (-)-58, white crystal; yield: 0.748g (80.1%); m.p.131.5 -132.0°C; (c 0.2, CH 3 OH); 1 H NMR (500MHz, Chloroform-d) δ8.18–8.13(m,2H),7.70–7.64(m,2H),7.59–7.53(m,2H),7.48–7.41(m,1H),7.32–7.23 (m,2H),7.21...
Embodiment 3
[0105] The preparation of embodiment 3 compound (+)-58-1 and (-)-58-1
[0106] (-)-58-1:
[0107]
[0108] White needle-like crystals; yield: 0.508g (75.4%); m.p.176.3-177.1℃; (c 0.2, CH 3 OH); 1 H NMR (500MHz, Chloroform-d) δ7.55–7.49(m,2H),7.42(ddt,J=8.7,7.1,1.2Hz,1H),7.31–7.22(m,2H),7.15–7.10(m ,2H),7.10–6.98(m,3H),6.18–6.12(m,1H),5.44(d,J=8.8Hz,1H),4.03(dd,J=9.8,8.9Hz,1H),2.90( d,J=0.6Hz,3H),2.09(s,3H); 13 C NMR (126MHz, Chloroform-d) δ197.17, 171.03, 169.99, 136.46, 133.75, 133.38, 128.78, 128.76, 128.55, 128.26, 72.21, 64.88, 49.16, 29.95, 21.02; HRMS ([ESI): m / z 1] + Calculated 338.1387Found 338.1389; The enantiomeric excess was determined to be 99.9% by HPLC with a Daicel Chiralcel AD-H column (4.6mm×25cm) (n-hexane / i-PrOH=80 / 20, λ=254nm, 1mL / min) ,t=9.4min.
[0109] (+)-58-1:
[0110]
[0111] White needle-like crystals; yield: 0.494g (73.4%); m.p.178.1-179.3°C; (c0.2, CH 3 OH); 1 H NMR (500MHz, Chloroform-d) δ7.55–7.49(m,2H),7.46–7.39(m,1H),7.29–7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com