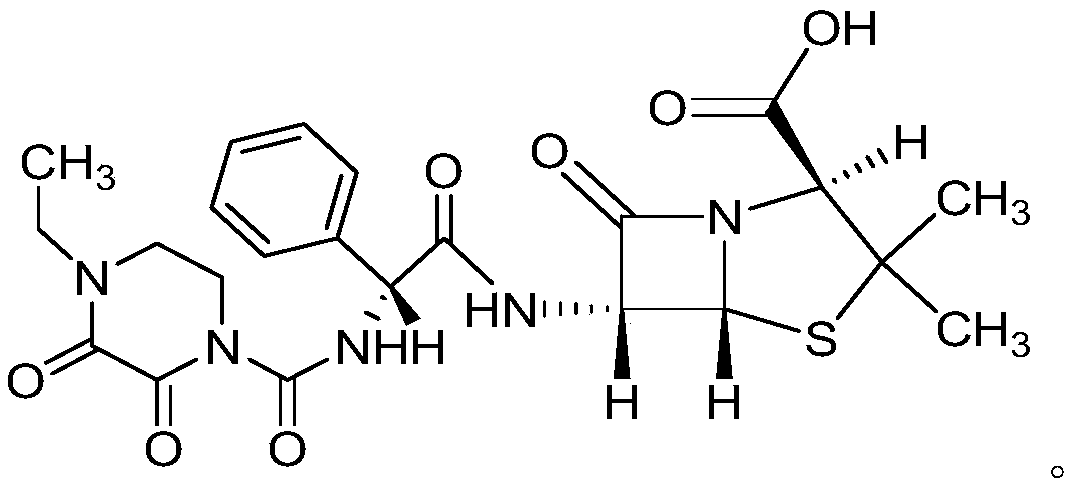
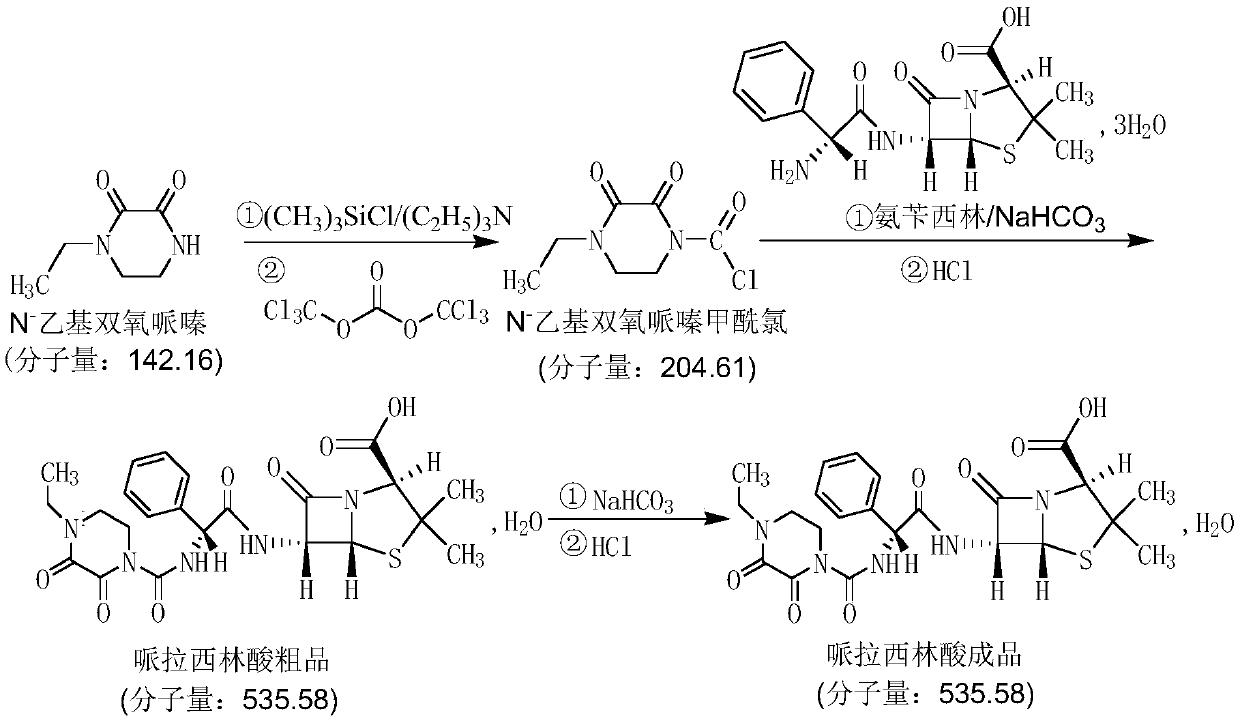
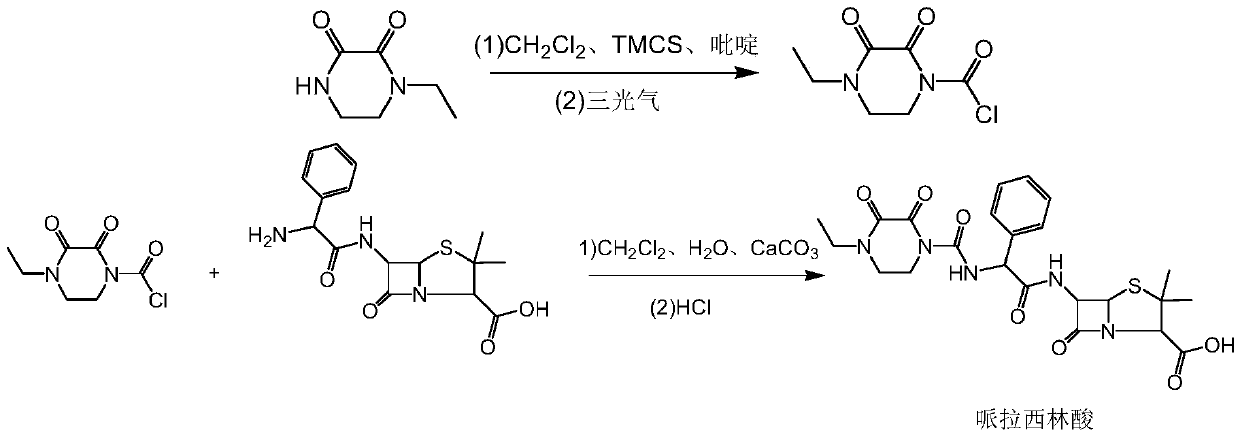
Preparation method of piperacillin acid
A technology of piperacillin acid and ethyldioxypiperazine, applied in the field of medicine, can solve the problems of low conversion rate of N-ethyldioxypiperazine, large loss of solvent, etc., and achieve high product yield and degraded impurity content. Low, conversion-increasing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: (after improvement)
[0029] 1) Take 14.2g (0.10mol) of N-ethyl dioxypiperazine, transfer it into a 500mL three-necked flask, stir, add 200mL of dichloromethane, cool down to -25~-20°C, add dropwise 16.3g (0.15mol) of TMCS , temperature control -25~-20℃, dropwise add 11.9g (0.15mol) pyridine, add 0.018g DMAP, temperature control -25~-20℃, add 11.9g (0.04mol) triphosgene in batches, keep warm for 30-60 minutes Carry out the reaction; after the reaction is completed, filter with suction, wash with 30 mL of dichloromethane, distill to dryness under reduced pressure, add 100 mL of n-hexane to crystallize, filter with suction, and dry to obtain 19.3 g of N-ethyldioxypiperazine chloride, with a yield of 94.3% .
[0030] 2) Transfer 20.2g (0.05mol) of ampicillin into a 500mL three-necked flask, add 100mL of water, 150mL of ethyl acetate, stir, add 5.0g of calcium carbonate, control the temperature at 30-35°C, and drop (12.3g of N-ethylbis Oxypiperazinyl chlorid...
Embodiment 2
[0031] Embodiment 2 (after improvement)
[0032] 1) Take 14.2g (0.10mol) of N-ethyl dioxypiperazine, transfer it into a 500mL three-necked flask, stir, add 200mL of dichloromethane, cool down to -25~-20°C, and dropwise add 16.50g (0.152mol) of TMCS , temperature control -25~-20°C, add 12.0g (0.152mol) pyridine dropwise, add 0.02g DMAP, temperature control -25~-20°C, add 11.9g (0.04mol) triphosgene in batches, keep warm for 30-60 minutes, Suction filtration, washing with 30 mL of dichloromethane, distillation under reduced pressure to dryness, crystallization by adding 100 mL of n-hexane, suction filtration, drying to obtain 19.2 g of N-ethyldioxypiperazine chloride, yield 93.8%.
[0033] 2) Transfer 20.2g (0.05mol) of ampicillin into a 500mL three-necked flask, add 100mL of water and 150mL of ethyl acetate, stir, add 5.2g of calcium carbonate, control the temperature at 30-35°C, and drop (12.3g of N-ethylbis Oxypiperazinyl chloride + 100mL dichloromethane) solution, dripping ...
Embodiment 3
[0034] Embodiment 3 (after improvement)
[0035] 1) Take 14.2g (0.10mol) of N-ethyl dioxypiperazine, transfer it into a 500mL three-necked flask, stir, add 200mL of dichloromethane, cool down to -25~-20°C, add dropwise 16.0g (0.147mol) of TMCS , temperature control -25~-20℃, dropwise add 11.6g (0.147mol) pyridine, add 0.02g DMAP, temperature control -25~-20℃, add 11.9g (0.04mol) triphosgene in batches, keep warm for 30-60 minutes , filtered with suction, washed with 30 mL of dichloromethane, distilled to dryness under reduced pressure, added 100 mL of n-hexane for crystallization, filtered with suction, and dried to obtain 19.1 g of N-ethyldioxypiperazine chloride, with a yield of 93.5%.
[0036] 2) Transfer 20.2g (0.05mol) of ampicillin into a 500mL three-necked flask, add 100mL of water, 150mL of ethyl acetate, stir, add 5.3g of calcium carbonate, control the temperature at 35-40°C, add dropwise (12.3g of N-ethylbis Oxypiperazinyl chloride + 100mL dichloromethane) solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com