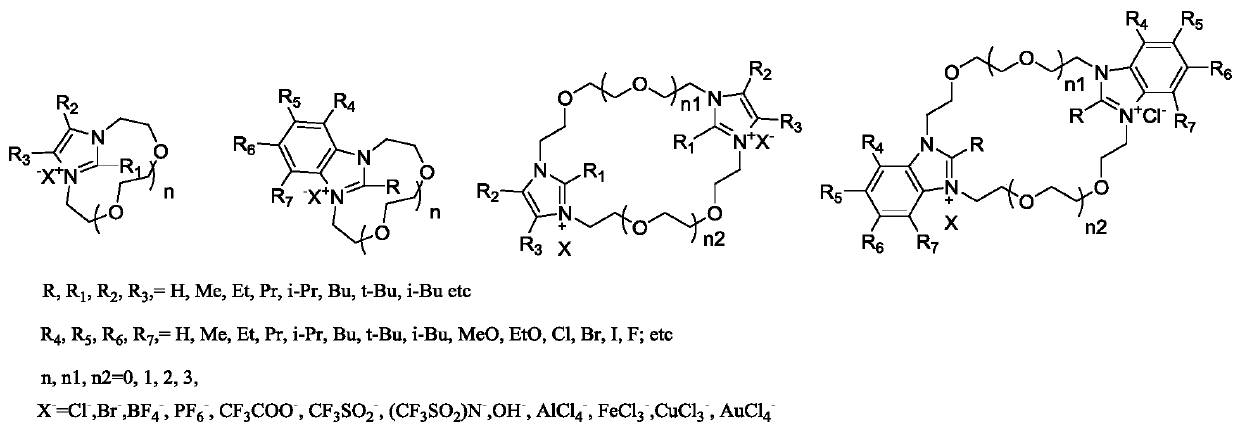
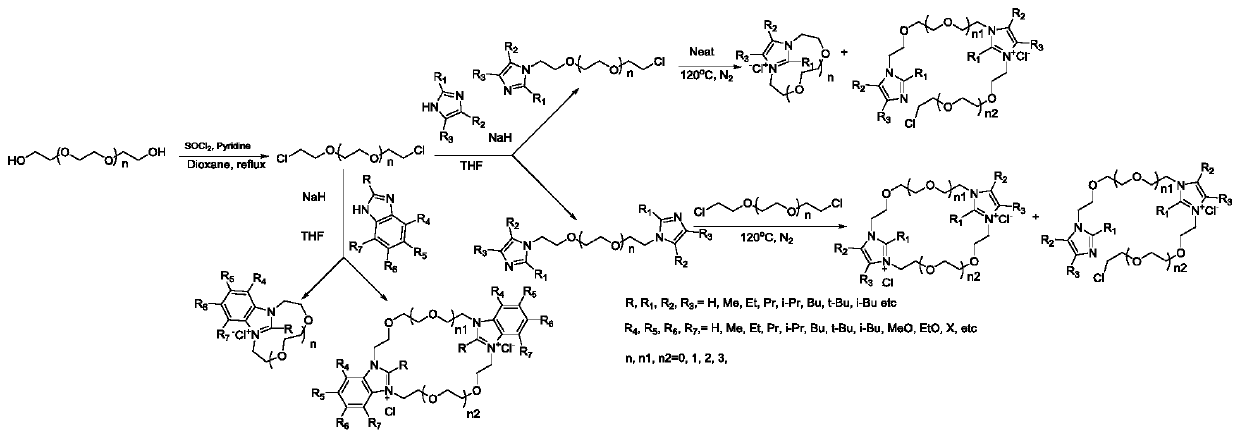
Crown ether type ionic liquid based on imidazole, benzimidazole and their derivatives
A benzimidazole and ionic liquid technology, applied in the field of ionic liquid preparation, can solve problems such as differences in extraction effects, and achieve the effects of fast reaction rate, high conversion rate, and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042]Further, the present invention also provides a method for preparing a crown ether-type ionic liquid based on imidazole, benzimidazole and derivatives thereof, which is characterized in that it comprises the following steps:
[0043] S1, the substitution reaction product of imidazole, benzimidazole and derivatives thereof and the acetonitrile that dichloroglycol is added are mixed, wherein the substitution reaction product of imidazole, benzimidazole and derivatives thereof is mixed with dichloroglycol and acetonitrile The molecular molar ratio is 1:0.9~1.1:18~22;
[0044] S2, heating the product obtained in step S1 to 55-65° C. under nitrogen protection, and reacting for 4-8 hours;
[0045] S3, concentrated, washed with ethyl acetate, and then removed the ethyl acetate layer;
[0046] S4. Separating and purifying the product in step S3 through a column to obtain an ionic liquid.
[0047] Further, the present invention also provides a method for preparing a crown ether-...
Embodiment
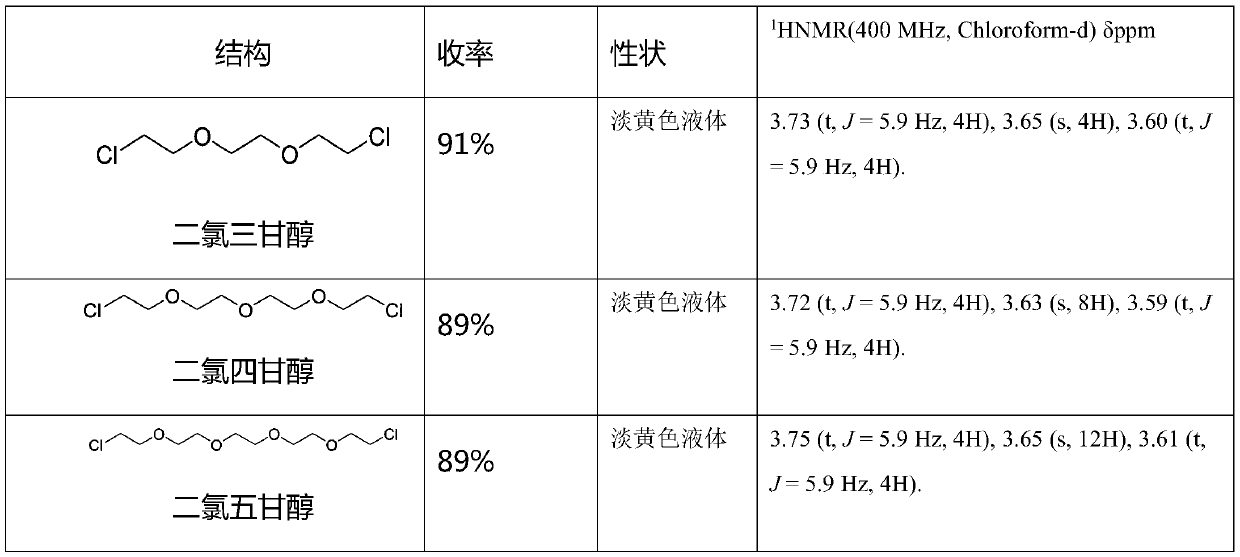
[0072] The preparation of dichloroglycol specifically comprises the following steps:
[0073] S1. Dissolve 1mol of diol and 3mol of pyridine into 500mL of dioxane, and heat to 80°C;
[0074] S2. Add 2.2 mol of thionyl chloride dropwise to the product in step S1, drop it slowly, and complete the dropwise addition in 2-3 hours. After the addition, react at a temperature of 80° C. for 5-6 hours;
[0075] S3, naturally cooling the product solution in step S2 to room temperature, distilling dioxane;
[0076] S4, adding the residue in step S3 to ethyl acetate, stirring for 30min, and filtering out the pyridinium salt;
[0077] S5. Wash the filtrate in step S4 with water, dry the ethyl acetate layer with anhydrous sodium sulfate, and concentrate to obtain a light yellow liquid.
[0078] Yield and spectrum analysis
[0079]
[0080] The preparation of the substitution reaction product of imidazole, benzimidazole and derivatives thereof specifically comprises the following steps:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com