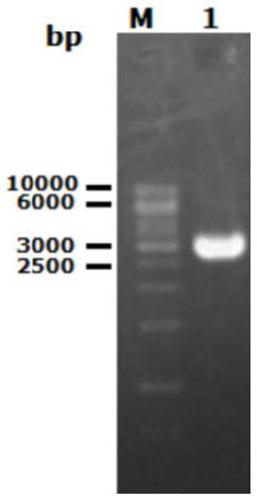
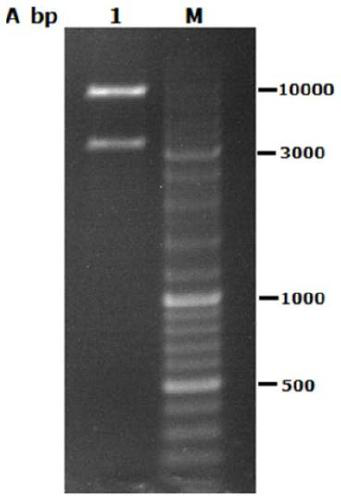
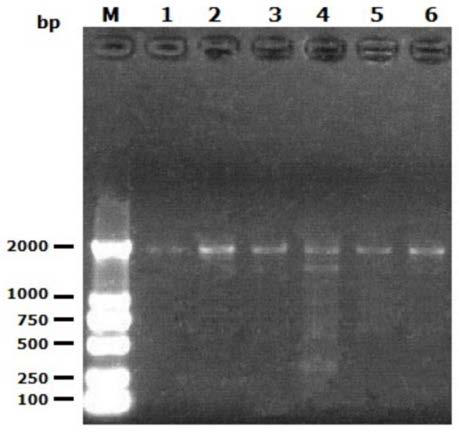
Method for expressing and purifying low temperature chitinase gene chiA in kluyveromyceslactis
A technology of chitinase gene and Kluyveromyces, which is applied in the fields of microbiology, enzyme engineering, fermentation engineering, and genetic engineering, can solve the problems of poor stability of chitinase and high cost of separation and purification, and reduce the separation Purification cost, effect of improving expression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Construction of Kluyveromyces lactis expression plasmid pKLAC2-chiA
[0039] 1.1 Primer design and amplification of target gene
[0040] According to the low-temperature chitinase chiA (GenBank No.KF234015) gene sequence and the codon preference of Kluyveromyces lactis, the signal peptide sequence was removed, and the gene mature peptide nucleotide sequence was codon-optimized using GeneOptimizer software, codon optimization The rear opt-chiA gene sequence is shown in SEQ ID NO.1. (GenBank No. MK635352).
[0041] Using the codon-optimized gene sequence of opt-chiA as a template, use Primer5.0 software to optimize the parameters of PCR primers, and design primers with XhoI and EcoRI restriction enzyme sites and protective bases. by TaKaRa LA Taq TM DNA Polymerase PCR amplifies the mature target fragment without signal peptide, and introduces a 6×His affinity purification tag at the C-terminus. The PCR reaction conditions were: 98°C for 1 min, 1 cycle; 98°C...
Embodiment 2
[0051] Example 2 Secretion and expression of low temperature chitinase gene chiA in Kluyveromyces lactis GG799
[0052] 2.1 Preparation of Kluyveromyces lactis GG799 (preserved in the laboratory) electroporation competent cells and their electroporation transformation
[0053] (1) pick fresh single colony of yeast in 5ml YPD liquid culture medium composed of 1% yeast powder, 2% peptone, and 2% glucose, and cultivate overnight at 30°C at 200rpm;
[0054] (2) Take 500 μl of the culture and inoculate it into a 250 mL Erlenmeyer shaker flask containing 50 ml of fresh YPD liquid medium, culture at 30°C and 200 rpm overnight until the OD600 reaches 1.3-1.5;
[0055] (3) Centrifuge the cell culture at 1500g for 5min at 4°C, and resuspend the cell pellet with 20mL of ice-cold sterile water;
[0056] (4) Centrifuge according to step 3, and resuspend the bacterial cell pellet with 20ml of ice-cold sterile water;
[0057] (5) Centrifuge according to step 3, and resuspend the bacterial ...
Embodiment 3
[0089] Example 3 Optimum Temperature and Temperature Stability, Optimum pH and pH Stability Research of Recombinant Chitinase ChiA
[0090] Effect of temperature on the enzyme ChiA
[0091] (1) Optimum reaction temperature: under the condition of pH value 8.0 (50mmol / L Tris-HCl buffer solution), with 4MU-(GlcNAc)2 as the substrate, the enzyme is determined within the temperature range of 4°C to 70°C According to the relative activity of the enzyme at different temperatures, draw a curve to determine the optimum reaction temperature of the enzyme.
[0092] (2) Thermal stability of the enzyme: the enzyme solution was incubated at different temperatures (4°C to 70°C) for 1 hour, and then the residual enzyme activity was detected at the optimum reaction pH and temperature, and compared with untreated Enzyme activity was compared to calculate relative activity.
[0093] Effect of pH on Enzyme ChiA
[0094] (1) Optimum reaction pH value: the enzyme activity reaction temperature i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| capacitance | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com