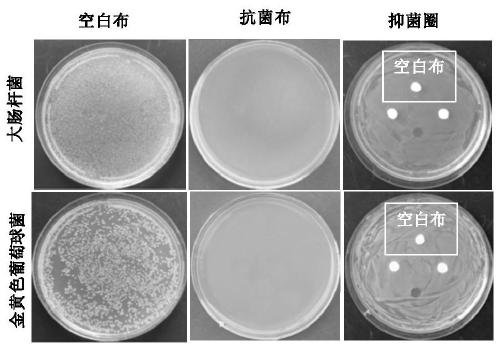
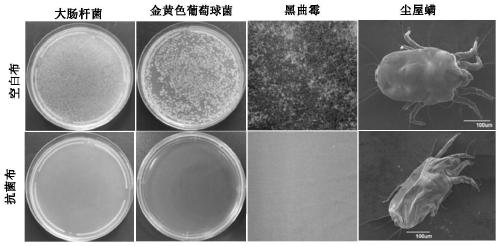
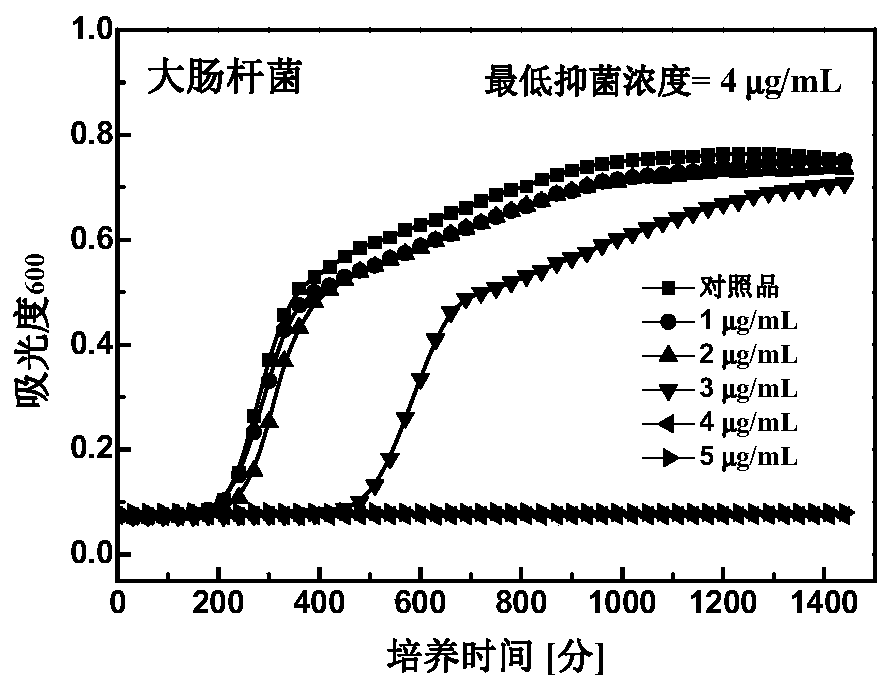
Compound, antibacterial finishing liquid, and preparation method and application of compound
An antibacterial finishing solution and compound technology, applied in the compound field, can solve the problems of fabric damage, unsatisfactory antibacterial effect, poor antibacterial activity persistence, etc., to improve softness and antistatic property, good antibacterial washability, good The effect of antibacterial, anti-mildew and anti-mite effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] The preparation process of the following examples 1 to 16 is carried out in two steps, and the reaction scheme is as follows:
[0142] A+B→Intermediate I
[0143] Intermediate I+D→E.
[0144] The structural formulas of reactants A, B, intermediate I, reactant D and product E in the examples are shown in Tables 1 and 2, respectively.
[0145] Table 1
[0146]
[0147]
[0148]
[0149] Table 2
[0150]
[0151]
[0152]
[0153] The specific reaction conditions for each example are as follows.
Embodiment 1
[0155] Add 1.35g (15mmol) of N,N-dimethylethanolamine (DMEA, compound A) and 4.35g (22.5mmol) of bromooctane (compound B) into a round bottom flask, and place it in an oil bath at 45°C React in medium for 2 hours, measure 12 mL of acetone as a solvent and add it to a round-bottomed flask, and then reflux for 12 hours; after the reaction, dry the solvent under reduced pressure at 60°C to obtain a white solid, which is washed several times with anhydrous petroleum ether, and placed in a vacuum oven Dry to obtain intermediate I, which is stored in a bottle for later use. The yield of this intermediate is 92%, and the nuclear magnetic resonance data is: 1H NMR (600MHz, D 2 O)δ3.92(tt, J=4.9,2.2Hz,2H),3.41–3.33(m,2H),3.29–3.22(m,2H),3.01(s,6H),1.66(ddd,J=15.0 ,9.4,5.6Hz,2H), 1.32–1.10(m,10H),0.75(t,J=6.8Hz,3H).
[0156] Take intermediate I, 1.42g (5mmol) and sodium carbonate 0.8g are dissolved in 10mL deionized water to configure a solution and add it to the dropping funnel for us...
Embodiment 2
[0158] Add 1.35g (15mmol) of N,N-dimethylethanolamine (DMEA, compound A) and 9.13g (22.5mmol) of compound B (bromooctadecane) into a round bottom flask, and place in an oil bath at 35°C React in the pot for 5 hours, measure 20 mL of ethanol as a solvent and add it to the round bottom flask, and then reflux for 8 hours; after the reaction, spin the solvent under reduced pressure at 40 ° C to obtain a white solid, wash it with anhydrous ether several times, and put it in a vacuum oven Oven dry, obtain intermediate (quaternary ammonium salt alcohol, i.e. intermediate I) and preserve in the bottle for use, the productive rate of this intermediate is 92%, and nuclear magnetic resonance data is: 1 H NMR (600MHz,D 2 O)δ3.92(tt, J=4.9,2.2Hz,2H),3.41–3.33(m,2H),3.29–3.22(m,2H),3.01(s,6H),1.66(ddd,J=15.0 ,9.4,5.6Hz,2H), 1.32–1.10(m,30H),0.75(t,J=6.8Hz,3H).
[0159] Take 4.22g (10mmol) of the intermediate (quaternary ammonium salt alcohol) and 1.3g (10mmol) of N,N-diisopropylethylamine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com