Near infrared zinc ion fluorescent probe compound, and preparation method and applications thereof
A fluorescent probe and zinc ion technology, which is applied in the field of fluorescent probes for metal ion detection, can solve the problems of long reaction time, poor selectivity of zinc ion fluorescent probes, poor water solubility, etc., and achieve short response time and strong anti-interference ability , easy to store effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Preparation of (E)-2-(3-(3-formyl-4-hydroxy)vinyl)-5,5-dimethylcyclohex-2-en-1-yl)malononitrile (formula ⅡIntermediate)
[0053] To a 100mL round bottom flask was added malononitrile (1.87g, 28.3mmol), isophorone (3.9g, 28.3mmol), N,N-dimethylformamide (10mL), piperidine (380μL, 3.84mmol), acetic acid (28μL, 0.19mmol), acetic anhydride (18μL, 0.5mmol), stir at room temperature for 1~2h, continue stirring at 80~85℃ for 1~2h, and then add p-hydroxybenzaldehyde ( 3.45g, 28.7mmol), stirred at 80~85℃ for 1~2h, while hot, pour the reactant into 200~220mL water containing 6~8mL concentrated hydrochloric acid, stir, filter with suction, and wash the solid with deionized water 6~ 8 times, the obtained solid was recrystallized with acetonitrile to obtain (E)-2-(3-(4-hydroxystyryl)-5,5-dimethylcyclohex-2-en-1-ylidene)propane Dinitrile (Intermediate of Formula IV).
[0054] Add (E)-2-(3-(4-hydroxystyryl)-5,5-dimethylcyclohex-2-en-1-ylidene)malononitrile (1.16g) to a 100mL round b...
Embodiment 2
[0062] The near-infrared zinc ion fluorescent probe compound described in Example 1 was used for testing.
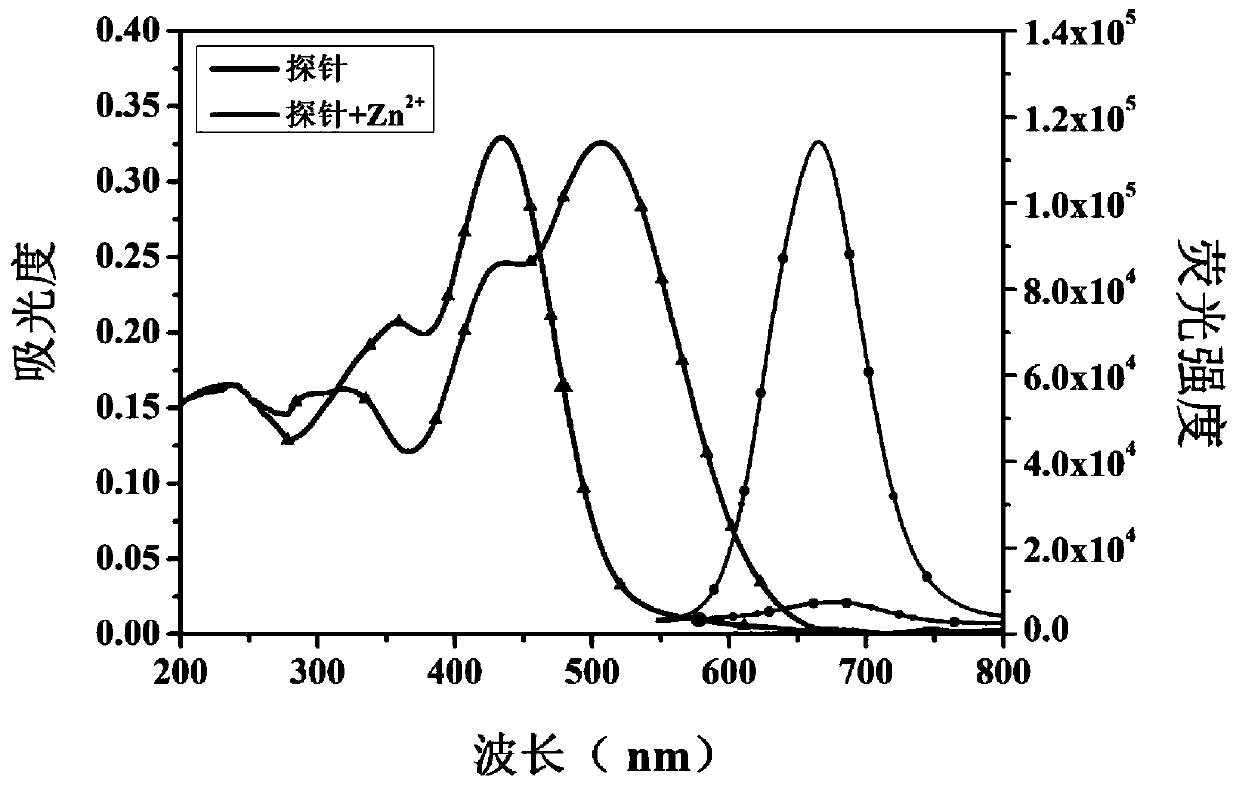
[0063] Dissolve the near-infrared zinc ion fluorescent probe compound in DMSO-H 2 In O(v / v=3:2) solution, the concentration is 10μM, and the concentration of other metal ions is 50μM. Test with fluorescence spectrometer and ultraviolet spectrophotometer. The maximum excitation wavelength of the fluorescent probe is 502nm and the maximum emission wavelength is 670nm. .
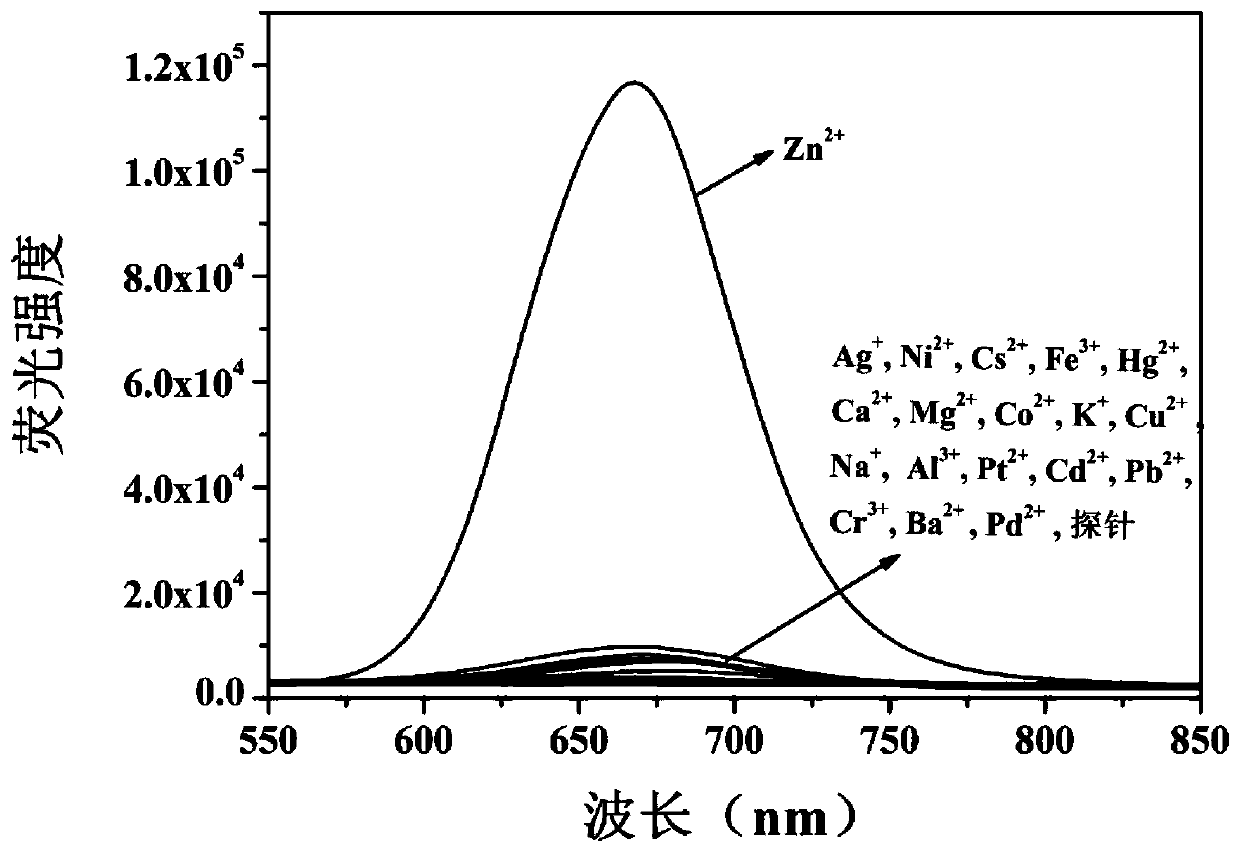
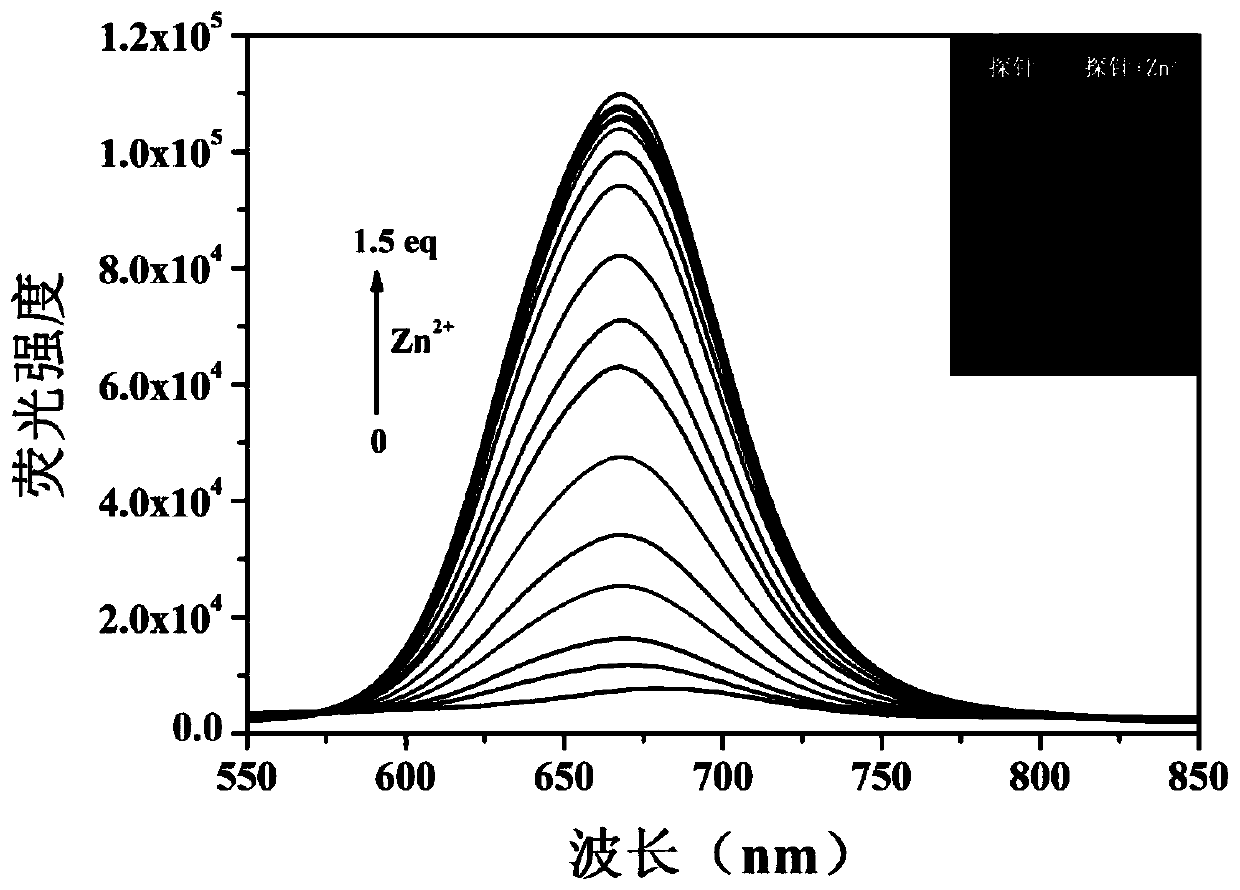
[0064] Such as figure 1 , Adding zinc ions (Zn 2+ ) UV absorption and fluorescence spectra before and after, the probe compound itself has an UV absorption peak at 434nm, but after the probe compound molecule is complexed with zinc ions, a new UV absorption peak appears at 502nm, and the probe compound itself is at 670nm There is almost no fluorescence, but after the probe compound molecule is complexed with zinc ions, it produces strong fluorescence at 670nm, in the near-infrared region, and the Stokes shift...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com