Preparation method of fluorescent probe for distinguishing peroxynitrite and hypochlorite on basis of xanthene and coumarin
A nitroso peroxide, fluorescent probe technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, fluorescence/phosphorescence, etc., can solve problems such as insufficient discrimination and achieve fast response time , high sensitivity, novel structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
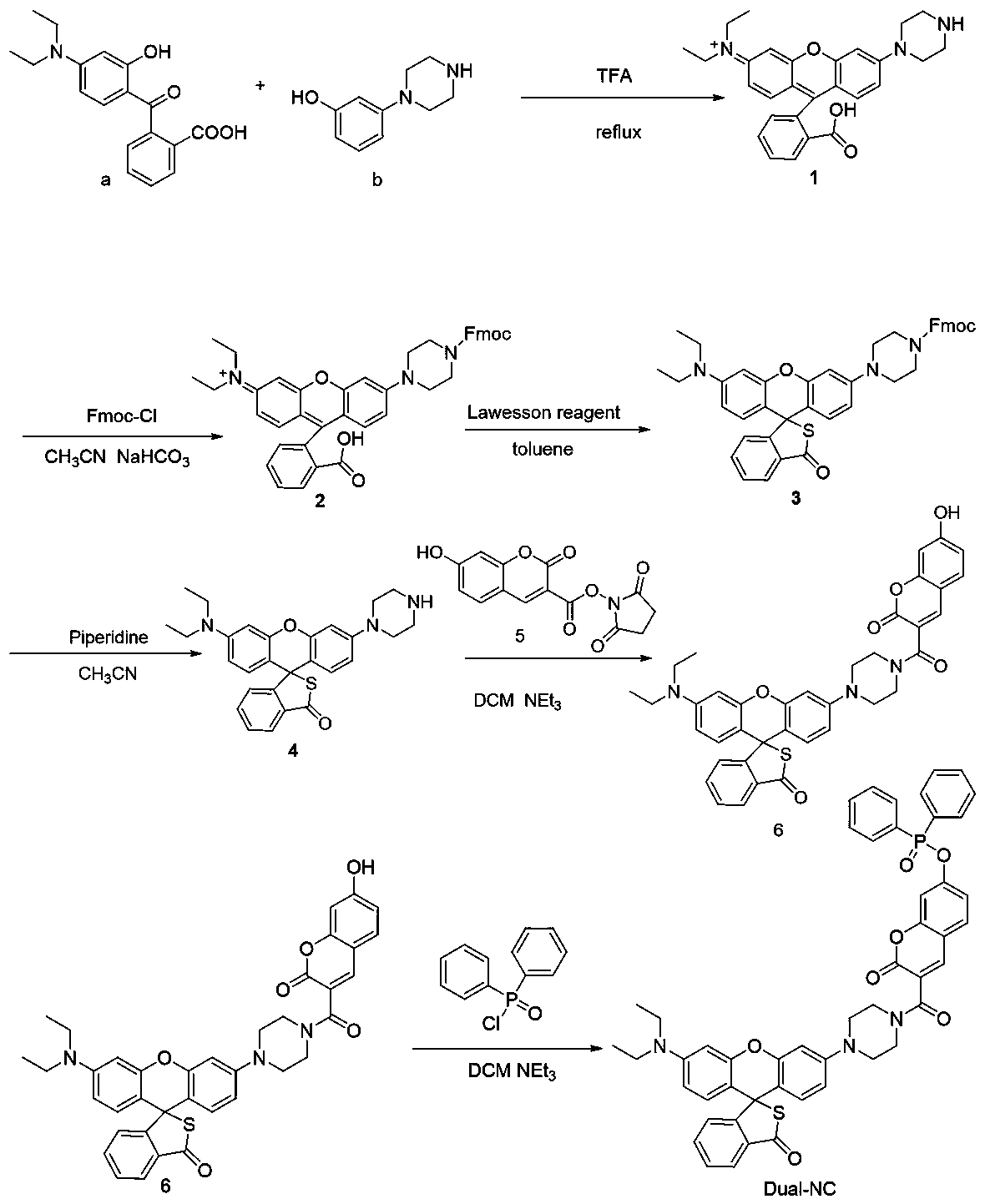
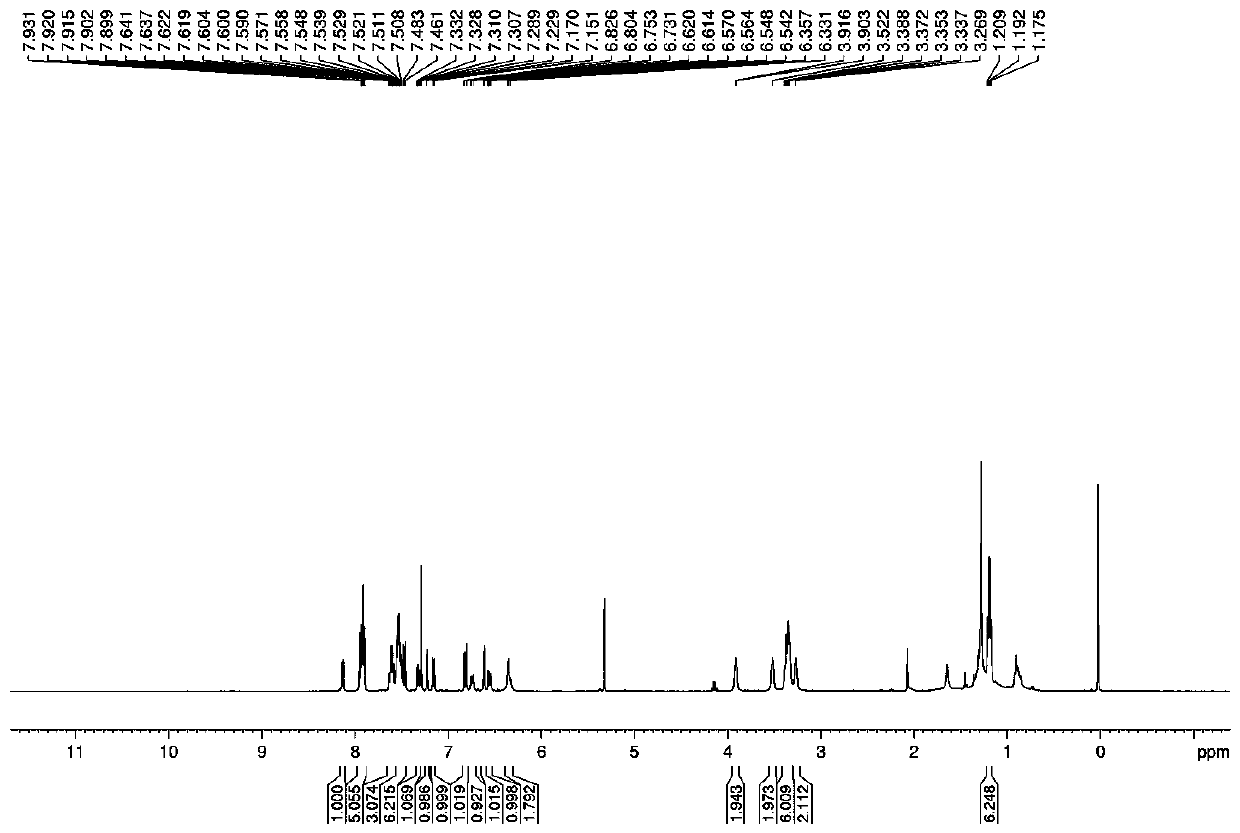
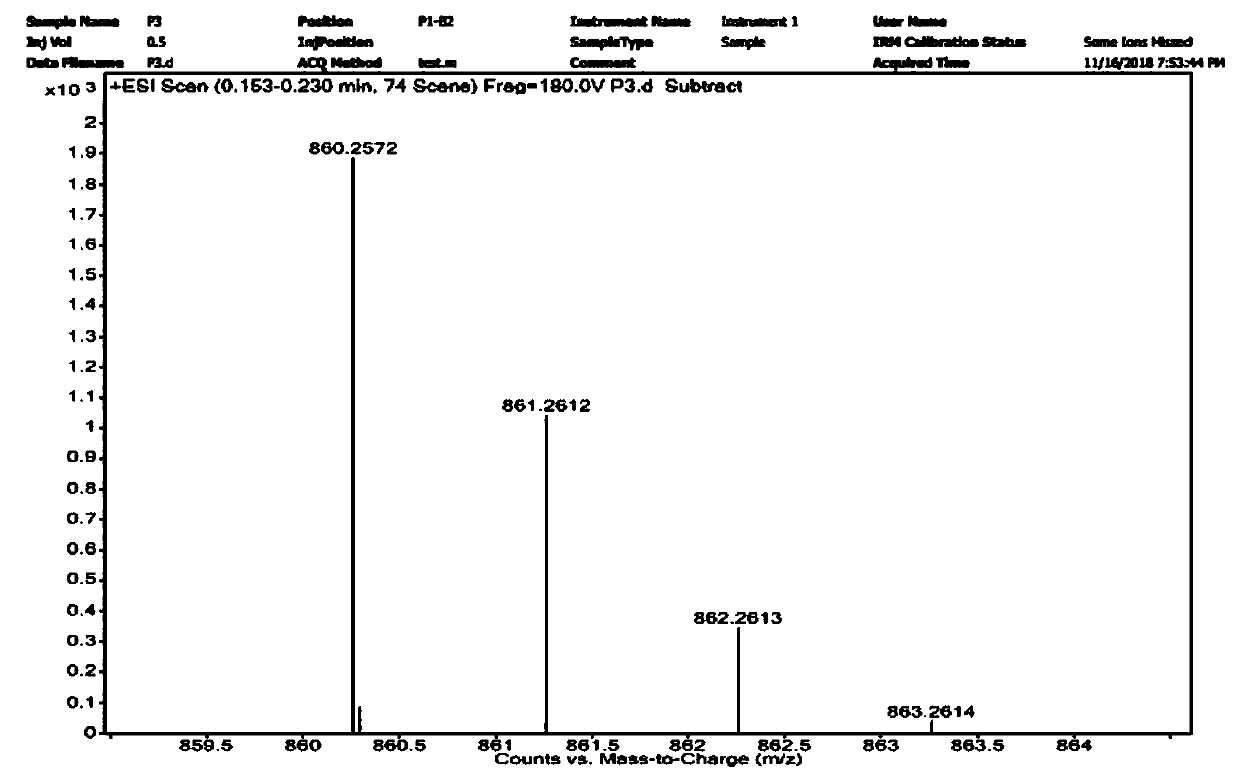
[0045] Embodiment 1: the synthesis of fluorescent probe Dual-NC
[0046] Dissolve 10mmol of compound a and 10mmol of compound b in 50mL of trifluoroacetic acid, heat and reflux at 80°C for 18 hours, distill off trifluoroacetic acid under reduced pressure, add 100mL of ice water, adjust the pH to 12 with saturated potassium carbonate, and dichloromethane Extraction, the organic layer was dried with anhydrous sodium sulfate, the solvent was spin-dried under reduced pressure, and separated with a 200-400 mesh silica gel column, the eluent was dichloromethane:methanol=10:1 (volume ratio), and red solid compound 1 was obtained;
[0047] Dissolve 5mmol of compound 1 and 5mmol of fluorenylmethoxycarbonyl chloride in 50mL of anhydrous acetonitrile, stir until dissolved, add 10mmol of sodium bicarbonate, and react for 12h under the protection of argon. After the reaction is complete, filter the filtrate to remove the solvent under reduced pressure, and use Extracted with ethyl acetate,...
Embodiment 2
[0060] Embodiment 2: the synthesis of fluorescent probe Dual-NC
[0061] Dissolve 10mmol of compound a and 10mmol of compound b in 50mL of trifluoroacetic acid, heat and reflux at 80°C for 18 hours, distill off trifluoroacetic acid under reduced pressure, add 100mL of ice water, adjust the pH to 12 with saturated potassium carbonate, and dichloromethane Extraction, the organic layer was dried with anhydrous sodium sulfate, the solvent was spin-dried under reduced pressure, and separated with a 200-400 mesh silica gel column, the eluent was dichloromethane:methanol=10:1 (volume ratio), and red solid compound 1 was obtained;
[0062] Dissolve 5mmol of compound 1 and 15mmol of fluorenylmethoxycarbonyl chloride in 50mL of anhydrous acetonitrile, stir until dissolved, add 10mmol of sodium bicarbonate, and react for 18h under the protection of argon. After the reaction is complete, filter the filtrate to remove the solvent under reduced pressure, and use Extracted with ethyl acetate...
Embodiment 3
[0068] Embodiment 3: the synthesis of fluorescent probe Dual-NC
[0069] Dissolve 10mmol of compound a and 10mmol of compound b in 50mL of trifluoroacetic acid, heat and reflux at 80°C for 18 hours, distill off trifluoroacetic acid under reduced pressure, add 100mL of ice water, adjust the pH to 12 with saturated potassium carbonate, and dichloromethane Extraction, the organic layer was dried with anhydrous sodium sulfate, the solvent was spin-dried under reduced pressure, and separated with a 200-400 mesh silica gel column, the eluent was dichloromethane:methanol=10:1 (volume ratio), and red solid compound 1 was obtained;
[0070] Dissolve 5mmol of compound 1 and 25mmol of fluorenylmethoxycarbonyl chloride in 100mL of anhydrous acetonitrile, stir until dissolved, add 10mmol of sodium bicarbonate, and react for 24h under the protection of argon. After the reaction is complete, filter, and remove the solvent from the filtrate under reduced pressure. Extracted with ethyl acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com