Alkali metal ion modified carbon nitride catalyst, and preparation method and application thereof
A technology of alkali metal ions and alkali catalysts, applied in the direction of physical/chemical process catalysts, separation methods, chemical instruments and methods, etc., can solve the problems of graphite phase carbon nitride crystallinity decrease, carbon nitride main structure destruction, л-conjugation System decline and other problems, to achieve the effect of mild and controllable conditions, cheap raw materials, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
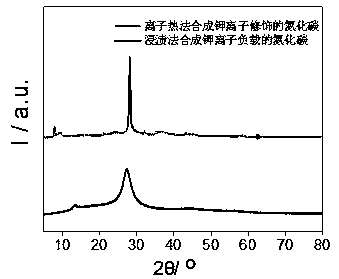
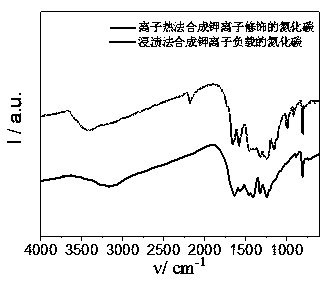
Image
Examples
Embodiment 1
[0027] Firstly, 10 g of melamine was weighed and placed in an alumina crucible, and calcined at 550° C. for 4 hours in an air atmosphere. After natural cooling, the sample was taken out and ground into powder to obtain carbon nitride powder. Weigh 2g of carbon nitride powder and 10g of alkali metal chloride (potassium chloride), grind evenly, and calcinate at 600°C for 2 hours in a nitrogen atmosphere, and the solid obtained after natural cooling is ultrasonically dispersed in water to remove free carbon nitride on the surface. Potassium ions, dried after suction filtration to obtain potassium ion-modified carbon nitride.
Embodiment 2
[0029] Firstly, 10 g of melamine was weighed and placed in an alumina crucible, and calcined at 550° C. for 2 h in an air atmosphere. After natural cooling, the sample was taken out and ground into powder to obtain carbon nitride powder. Weigh 2g of carbon nitride powder and 10g of alkali metal chloride (sodium chloride) to grind evenly, calcinate at 600°C for 2 hours in a nitrogen atmosphere, and the solid obtained after natural cooling is ultrasonically dispersed in water to remove free carbon nitride on the surface. sodium ions, and dried after suction filtration to obtain sodium ion-modified carbon nitride.
Embodiment 3
[0031] Firstly, 10 g of melamine was weighed and placed in an alumina crucible, and calcined at 550° C. for 4 hours in an air atmosphere. After natural cooling, the sample was taken out and ground into powder to obtain carbon nitride powder. Weigh 2g of carbon nitride powder and 10g of alkali metal chloride (5g of sodium chloride and 5g of potassium chloride) and grind evenly, calcinate at 600°C for 2 hours in a nitrogen atmosphere, and the solid obtained after natural cooling is ultrasonically dispersed in water to remove Sodium and potassium ions are free on the surface of the carbon nitride, and dried after suction filtration to obtain carbon nitride decorated with sodium and potassium ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com