A Class of Alkyl/Sulfanyl Aza-Heteroaromatic Terminations of d(a-ar) 2 Type conjugated compound and its preparation method and application
A technology of conjugated compound and sulfanyl nitrogen, which is applied in the field of D2-type conjugated compound and its preparation, can solve the problems of hindering the development of high-efficiency organic small molecule solar cells, low photoelectric conversion efficiency, etc., and achieves enhanced charge transport performance, The effect of good film formation and high carrier mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1, compound 2-octyl thiazole (TZR 1 ) synthetic route is as follows:
[0060]
[0061] Under nitrogen protection, in a 100mL there-necked flask, dissolve 2-bromothiazole (6.0g, 36.58 mmol) with dry ether, add dropwise 2.5M n-butyllithium (16.0ml, 40.24mmol), -78 After reacting at °C for two hours, n-octane bromide (7.0 g, 36.58 mmol) was added at one time, and after incubation for half an hour, the reaction was transferred to room temperature overnight. The solvent was directly spin-dried under reduced pressure to obtain a pale yellow liquid, which was separated by column chromatography with petroleum ether as an eluent to obtain the product (TZR 1 ) 6.0 g, 84% yield. 1 H NMR (500MHz, CDCl 3 )δ7.66(d, J=3.3Hz,1H),7.18(d,J=3.3Hz,1H),3.05–2.97(m,2H),1.79(dt,J=15.4,7.6 Hz,2H), 1.33–1.26(m, 11H), 0.87(s, 3H).
Embodiment 2
[0062] Example 2, 2-Octylthiazole-5-tributyltin (SnTZR 1 ) synthetic route is as follows:
[0063]
[0064] Under nitrogen protection, in a 100 mL three-necked flask, dissolve 2-octylthiazole (2.46 g, 10.8 mmol) with dry tetrahydrofuran, add 2.5M n-butyllithium (4.75 ml, 11.88 mmol) dropwise at -78°C, - After reacting at 78° C. for two hours, tributyltin chloride (3.22 ml, 11.88 mmol) was added at one time, and after the reaction was incubated for half an hour, the reaction was transferred to room temperature overnight. The solvent was directly spin-dried under reduced pressure to obtain a light yellow thick liquid (SnTZR 1 ), which was directly used in the next reaction. 1 H NMR (400MHz, CDCl 3 )δ7.58(s,1H),3.06–3.03(m,2H), 1.80(dt,J=15.0,7.4Hz,2H),1.58–1.51(m,6H),1.46(dt,J=16.1, 8.7Hz, 6H), 1.39-1.17(m, 22H), 0.89(t, J=7.2Hz, 12H).
Embodiment 3
[0065] Example 3,3-(5-Bromo-2-thienyl)-2,5-bis(2-ethylhexyl)-6-(5-(2-octyl-5-thiazolyl)-2-thiophene base) diketopyrrolopyrrole (BrTDPP-TZR 1 ) synthetic route is as follows:
[0066]
[0067] Under nitrogen protection, 20 mL of toluene, 2-octylthiazole-5-tributyltin (286 mg, 0.59 mmol), 3,6-bis(5-bromo-2-thienyl)-2,5- Bis(2-ethylhexyl)-pyrrolopyrrole dione (400 mg, 0.59 mmol), tetrakistriphenylphosphine palladium (34 mg, 0.03 mmol). The mixture was heated to 80°C with stirring under nitrogen atmosphere, the reaction was stopped after 4 h, and the mixture was cooled to room temperature. The solvent was removed by rotary evaporation, and the mixed solution whose volume ratio of petroleum ether / dichloroalkane was 5:1 was the eluent to carry out column chromatographic separation to obtain the product (BrTDPP-TZR 1 ) 172 mg, 30.0% yield. 1 H NMR (400MHz, CDCl 3)δ8.90(d,J=4.1Hz,1H), 8.64(d,J=4.2Hz,1H),7.84(s,1H),7.27(s,1H),7.23(d,J=4.2Hz, 1H), 3.98(dt, J=13.7, 7.2Hz, 4H), 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
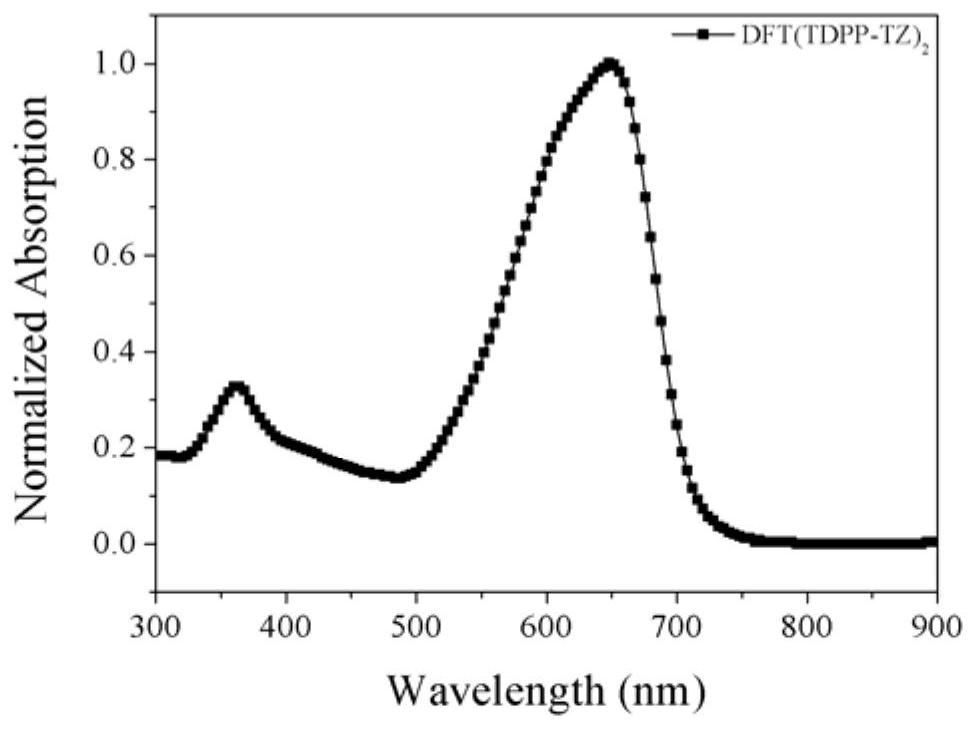
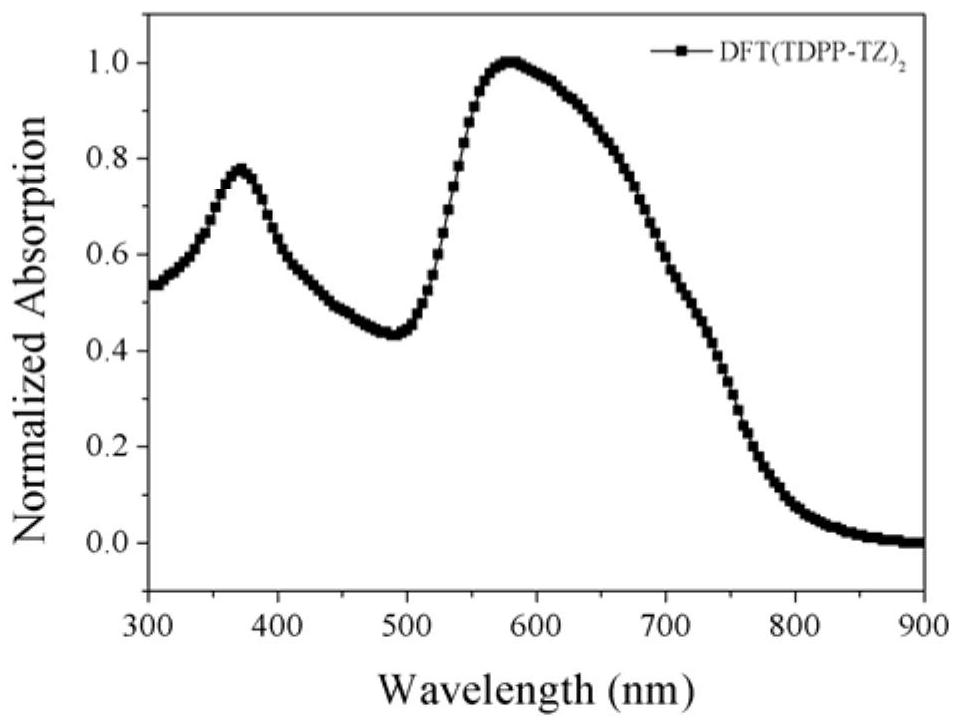
| thickness | aaaaa | aaaaa |
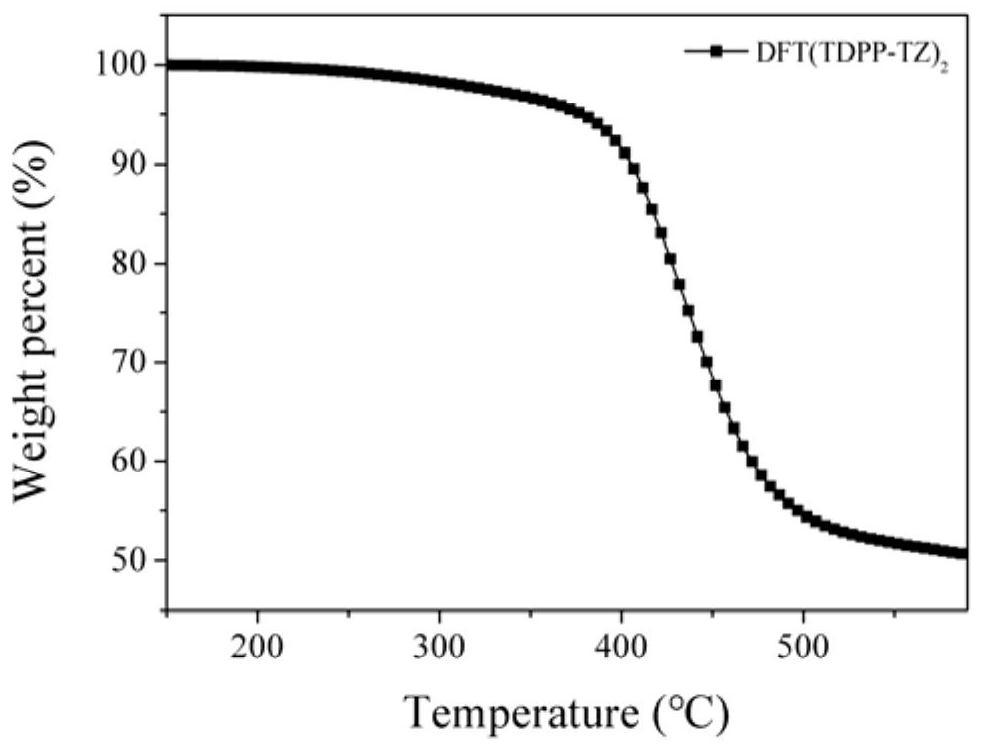
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com