Conjugated microporous polymer, and preparation method and application thereof
A technology of conjugated micropores and polymers, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, water pollutants, etc. Restrictions, difficulty in meeting the diversity of catalytic systems, etc., to achieve good solvent tolerance, economical improvement, and the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 compound II3, the synthesis of 6-dibromo-9-(4-bromophenyl)-9H-carbazole
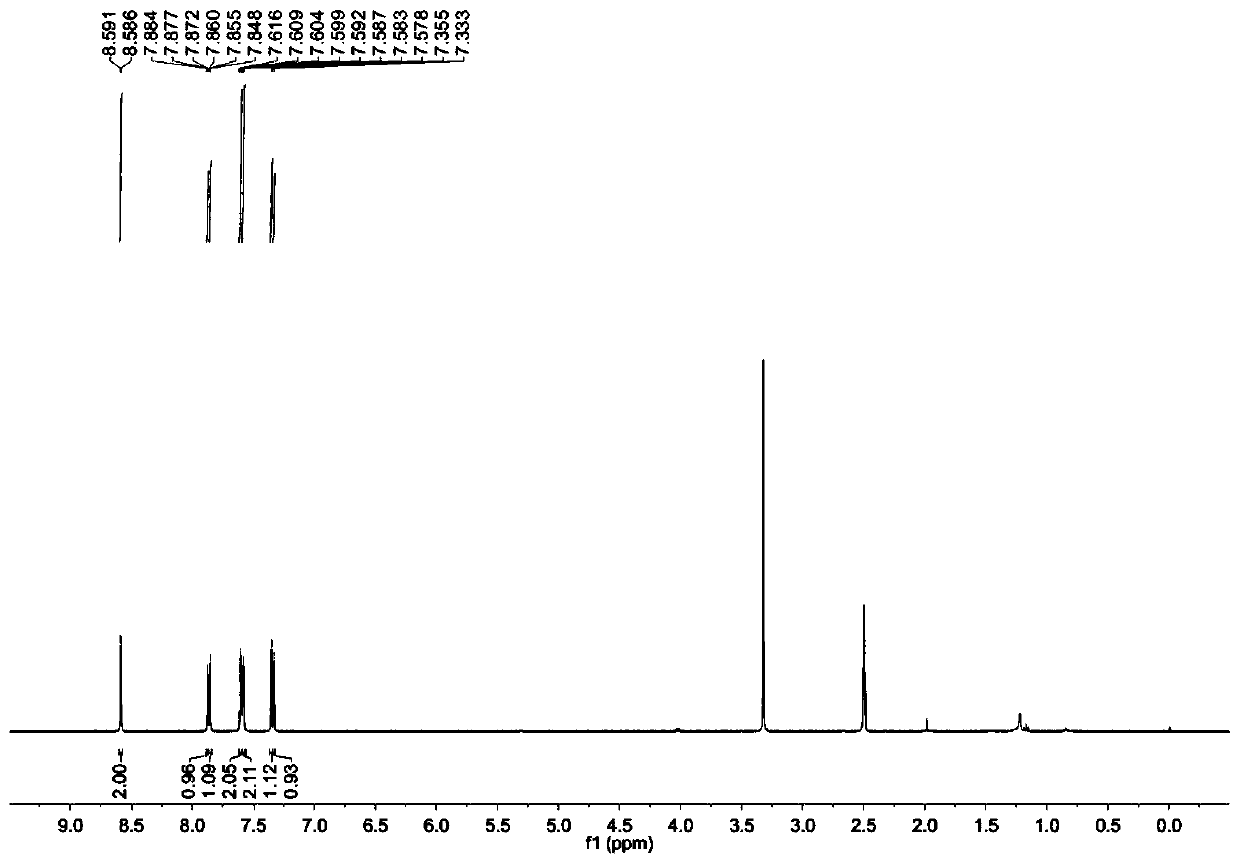
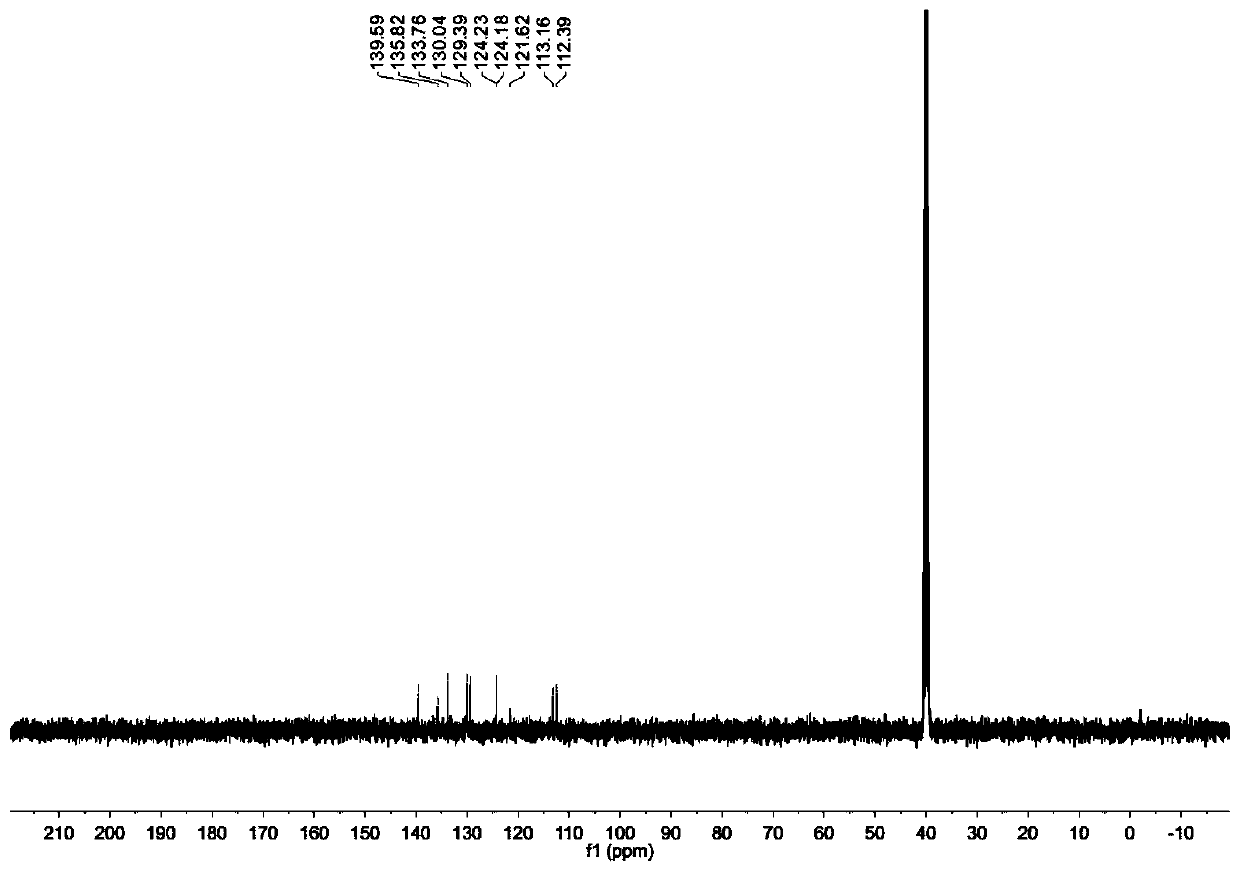
[0048] With 2.5g (7.7mmol) compound A1, Cs 2 CO 3 7.5g (23mmol), 4g (22.9mmol) of 1-bromo-4-fluorobenzene were mixed in a round bottom flask and 25mL of dimethyl sulfoxide was added, and heated at 120°C for 24 hours; the resulting mixture was introduced into 100mL of distilled water, The obtained solid was filtered out, dried and purified by column chromatography, the eluent was petroleum ether, and the obtained white solid compound was compound II (1.41 g, yield 45.1%). Picture 1-1 For the prepared compound II 1 H NMR spectrum, Figure 1-2 For the prepared compound II 13 C NMR spectrum. 1 H NMR (400MHz, DMSO-d6): δ=8.59(d,2H,J=2.0 Hz),7.88(m,1H),7.86(m,1H),7.61(m,2H),7.59(m,2H ),7.36(s,1H),7.33(s 1H). 13 C NMR (100MHz, DMSO-d6): δ = 139.59, 135.82, 133.76, 130.04, 129.39, 124.23, 124.18, 121.62, 113.16, 112.39.
[0049] The synthetic reaction formula of compound II is as fo...
Embodiment 2
[0051] Synthesis of embodiment 2 compound III 1,3,5-triethynylbenzene
[0052] 1. With 1.259g (4.0mmol) compound B1, 0.421g (0.6mmol) ditriphenylphosphine palladium dichloride, 0.158g (0.6mmol) triphenylphosphine, 0.114g (0.6mmol) cuprous iodide, Add 10mL tetrahydrofuran and 10mL diisopropylamine into a 100mL round bottom flask, then add 4.4mL (30mmol) trimethylsilylacetylene; stir the resulting mixture at 50°C for 24 hours; then spin out the solvent under reduced pressure and add dichloromethane and water for extraction and washing, the organic phase was dried with magnesium sulfate, and finally spin-dried. Purified by column chromatography, the eluent was petroleum ether: ethyl acetate = 10:1, and 1.061 g (84.3% yield) of compound B2 was obtained as a colorless solid. diagram 2-1 For the prepared compound B2 1 H NMR spectrum, Figure 2-2 For the prepared compound B2 13 C NMR spectrum. 1 H NMR (400MHz, DMSO-d6) δ: 7.49(s, 3H), 0.23(s, 27H). 13 C NMR (100MHz, CDCl 3 )...
Embodiment 3
[0056] Synthesis of Example 3 Conjugated Microporous Polymer SCUT-1
[0057] 57.2mg (0.381mmol) compound III, 122mg (0.254mmol) compound II, 43.89mg (0.038mmol) tetrakistriphenylphosphine palladium, 7.3mg (0.038mmol) cuprous iodide were added to 2mL di In a mixed solvent of isopropylamine / N,N-dimethylformamide (v / v=1:1), ultrasonically disperse for 3 minutes, freeze and deoxygenate 3 times, heat to 80°C, and react for 72 hours. Cool to room temperature, filter, wash with dichloromethane, acetone, methanol, and water successively, repeat 3 times to remove unreacted monomer and catalyst, and dry the obtained solid in vacuum at 80°C for 24 hours to obtain 101 mg of brown powder SCUT-1 (produced rate of 88%).
[0058]
[0059] Figure 4 Infrared spectra of the conjugated microporous polymer SCUT-1 prepared in Example 3 and its reactants, Compound II and Compound III. Comparing the infrared spectra of these three compounds, it can be seen that in the compound III at 3275cm -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com