Preparation method and application of D-A BODIPY conjugated polymer
A type of fluoroboron dipyrrole, conjugated polymer technology, applied in the field of solar cells, can solve the problems of disordered structure, poor durability, complex process, etc., to enhance the transfer of electrons and holes, increase the diffusion distance, and expand the light. The effect of the range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
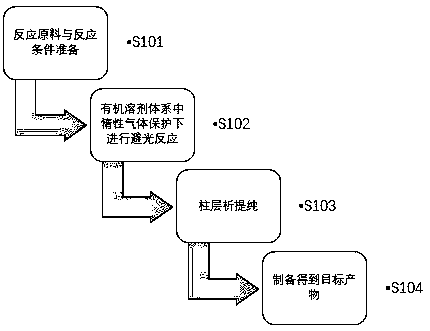
[0044] The first aspect of the present invention provides a method for preparing a D-A type fluoroboron dipyrrole conjugated polymer, such as figure 1 shown, including the following steps:
[0045] S101: Preparation of reaction raw materials and reaction conditions; the reaction raw materials are fluoroboron dipyrrole compounds (structural formulas are shown in formula I and formula II), electron-donating monomers, and catalysts;
[0046]S102: In an organic solvent system, under the protection of an inert gas, conduct a reaction in the dark;
[0047] S103: column chromatography purification;
[0048] S104: Prepare the target product (the structural formula is shown in formula III and formula IV)
[0049] The specific preparation method of the D-A type fluoroboron-dipyrrole conjugated polymer is as follows: the fluoroboron-dipyrrole compound is respectively reacted with an electron-donating monomer and a catalyst in an organic solvent system under the protection of an inert g...
Embodiment 1
[0072] The invention provides a method for preparing a D-A type fluoroboron dipyrrole conjugated polymer. 0.492 mmol of the compound represented by formula I and 0.738 mmol of 5,5'-bistrimethyltin-2,2'-bithiophene Place in dichloromethane solvent, use copper oxide, tris(2-tolyl)phosphine and tris(dibenzylideneacetone)dipalladium as catalysts, under the protection of inert gas, control the temperature at 110°C, and avoid light for 48h , and purified by column chromatography to obtain the target product formula III.
[0073] The synthetic route of the formula III is as follows:
[0074]
[0075] The NMR of the target product formula III is: 1 H NMR (CDCl 3 ,400MHz,δ / ppm): 7.55(br,1H), 7.14(br,3H), 7.02(br,2H), 6.78(br,1H), 2.62(br,6H), 1.58(br,6H); 13 C NMR (CDCl 3 , 100MHz, δ / ppm): 152.46, 142.85, 138.25, 135.13, 130.77, 123.56, 120.55, 118.47, 114.68, 76.15, 34.95, 33.85, 31.47, 29.54, 27.12, 16.67, 9.87.
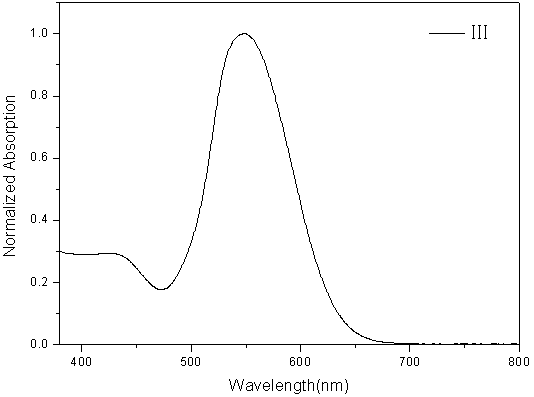
[0076] figure 2 The ultraviolet-visible absorption spectrum ...
Embodiment 2
[0086] The invention provides a method for preparing a D-A type fluoroboron dipyrrole conjugated polymer. 0.523 mmol of the compound represented by formula II and 0.628 mmol of 5,5'-bistrimethyltin-2,2'-bithiophene Place in dichloromethane solvent, use copper oxide as catalyst, under the protection of inert gas, control the temperature at 110°C, react in the dark for 48 hours, and purify by column chromatography to obtain the target product formula IV.
[0087] The synthetic route of described formula IV is as follows:
[0088]
[0089] The NMR of the target product formula IV is: 1 H NMR (CDCl 3 ,400MHz,δ / ppm): 7.55(br,1H), 7.14(br,3H), 7.02(br,2H), 6.78(br,1H), 2.62(br,6H), 1.58(br,6H); 13 C NMR (CDCl 3 , 100MHz, δ / ppm): 152.46, 142.85, 138.25, 135.13, 130.77, 123.56, 120.55, 118.47, 114.68, 76.15, 34.95, 33.85, 31.47, 29.54, 27.12, 16.67, 9.87.
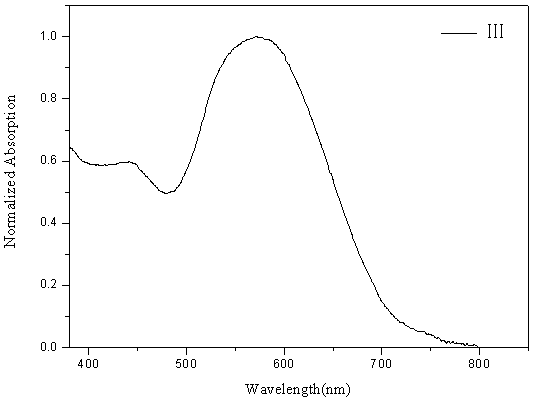
[0090] Figure 5 The ultraviolet-visible absorption spectrogram of the D-A type fluoroboron dipyrrole conjugated polymer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com