A kind of injectable PMMA antibiotic bone cement and preparation method thereof
A technology of antibiotics and bone cement, applied in tissue regeneration, prosthesis, medical science, etc., can solve the problems of volume shrinkage, poor biological activity, high modulus of antibiotic bone cement, etc., achieve efficient and sustained release, increase specific surface area and porosity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
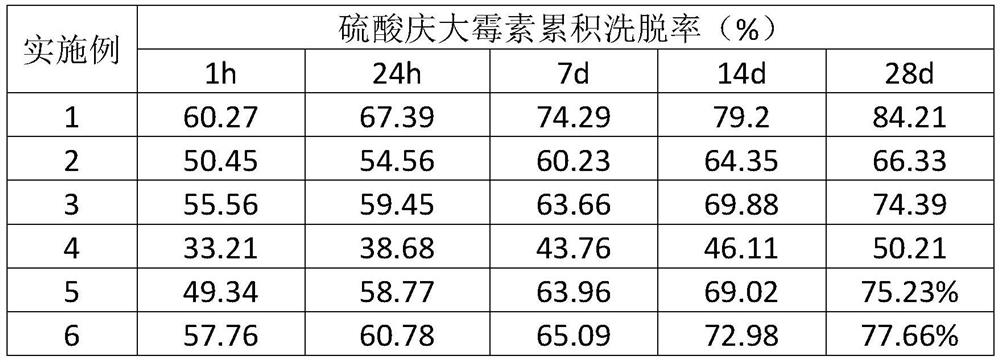
Examples
preparation example Construction
[0033] A kind of preparation method of injectable PMMA antibiotic bone cement of the present invention specifically comprises the following steps:
[0034] Step 1, preparing CMC-g-PAA copolymer powder;
[0035] The concrete steps of preparing CMC-g-PAA copolymer powder are:
[0036] Step 1.1: Take carboxymethyl cellulose 2.702%~15.151%, acrylic acid 54.054%~60.606%, initiator ammonium persulfate 0.2%~2%, crosslinking agent N,N-methylenebisacrylamide 0.02%~ 0.2% and deionized water 32.432% ~ 36.363%, total 100%. Add carboxymethyl cellulose solution, AA, cross-linking agent MBA solution and initiator APS solution to the four-necked flask in sequence and mix evenly. The obtained mixed solution is continuously stirred and heated to 35°C-55°C, in which the carboxymethyl The preparation methods of the cellulose solution, the initiator ammonium persulfate and the crosslinking agent N,N-methylenebisacrylamide are all dissolved in deionized water at room temperature, supplemented by ...
Embodiment 1
[0045] Step 1, the preparation of CMC-g-PAA copolymer:
[0046] The reaction was carried out in a four-necked flask equipped with a mechanical stirrer, a thermometer, a condensing reflux device and nitrogen gas. Take 6 g of carboxymethyl cellulose, 50 g of acrylic acid, 0.5 g of initiator ammonium persulfate, and crosslinking agent N, respectively. Add 0.05 g of N-methylenebisacrylamide and 30 g of deionized water, add carboxymethyl cellulose solution, AA, cross-linking agent MBA solution and initiator APS solution into a four-necked flask in sequence and heat to 55°C, continuously stirring, use N 2 Purging for 1h to remove dissolved oxygen in the solution, then raising the temperature to 75°C, and reacting at a constant temperature in a nitrogen atmosphere for 4h, the CMC-g-PAA copolymer can be obtained, and the obtained product is washed 20 times with deionized water, and then CMC-g-PAA copolymer powder was obtained by vacuum drying at ℃ for 24 h to constant weight, and fin...
Embodiment 2
[0054] Step 1, the preparation of CMC-g-PAA copolymer:
[0055] The reaction was carried out in a four-necked flask equipped with a mechanical stirrer, a thermometer, a condensing reflux device and nitrogen gas. Take 6 g of carboxymethyl cellulose, 50 g of acrylic acid, 0.05 g of initiator ammonium persulfate, and crosslinking agent N, respectively. 0.005g of N-methylenebisacrylamide and 30g of deionized water, add carboxymethyl cellulose solution, AA, crosslinking agent MBA solution and initiator APS solution into a four-necked flask in sequence and continue to stir and heat to 40°C. use N 2 Purging for 0.5h to remove dissolved oxygen in the solution, then raising the temperature to 70°C, and reacting at a constant temperature in a nitrogen atmosphere for 2h, the CMC-g-PAA copolymer can be obtained, and the obtained product is washed 15 times with deionized water, and then Vacuum-dried at 60° C. for 12 h to constant weight, and finally ground and sieved to obtain CMC-g-PAA c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com