Method for producing gamma-aminobutyric acid through catalysis of saccharomyces cerevisiae cells and gamma-aminobutyric acid
A technology of Saccharomyces cerevisiae cells and aminobutyric acid, which is applied in the field of γ-aminobutyric acid, can solve the problems of incomparable GABA yield, low GABA synthesis efficiency, complex components, etc., and achieves a perfect exogenous gene expression system, easy control, and benefits. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0051] Experimental Example 1: Expression and Characterization of SsGAD and ScGAD
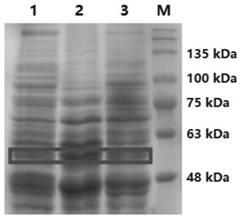
[0052] The SsGAD and ScGAD genes from MJ654-NF4 and the two genes of Streptomyces rufosa ATCC 49982 were amplified from the Streptomyces genomic DNA sample, and connected into the yeast expression vector YEpADH2p-TRP to produce pHY24 (YEpADH2p-TRP-SsGAD) respectively and pHY25(YEpADH2p-TRP-ScGAD), the GAD gene was expressed in Saccharomyces cerevisiae BJ5464 by an ADH promoter and an ADH terminator. SDS-PAGE analysis showed that SsGAD and ScGAD were successfully expressed in yeast, such as Figure 1a , the relative molecular weights of SsGAD and ScGAD were calculated to be 54.7kDa and 56.3kDa, respectively. Therefore, although these two GADs are originally from Streptomyces, the findings suggest that they can be heterologously expressed in S. cerevisiae BJ5464.
experiment example 2
[0053] Experimental Example 2: Biotransformation of L-GLU into GABA by Engineering Saccharomyces cerevisiae BJ5464
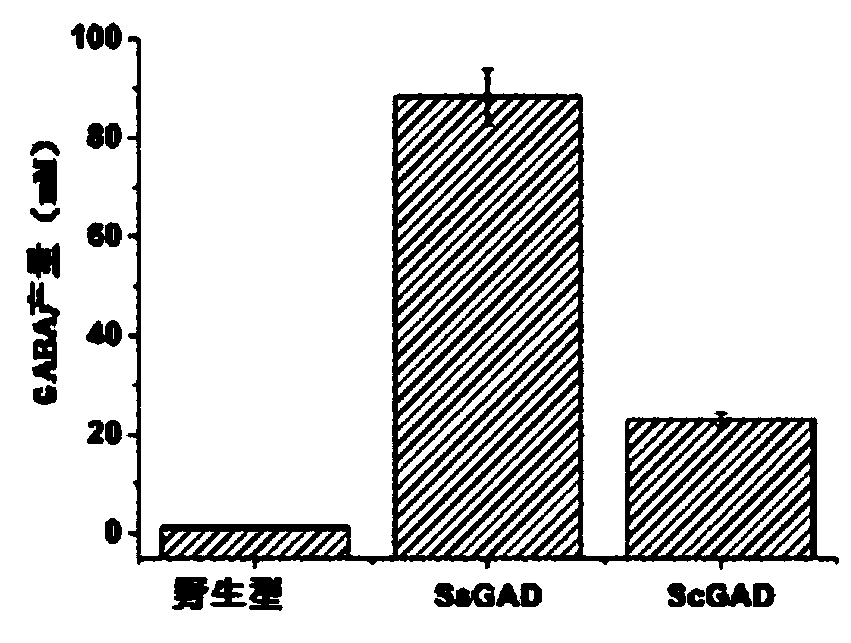
[0054] In order to prove whether the expressed SsGAD and ScGAD work in yeast, the following method was used to prove: the corresponding Saccharomyces cerevisiae engineering strain BJ5464 reacted with L-GLU, and the wild type was used as a control. The reaction mixture contained 0.1M L-GLU, 0.2mM PLP and yeast cells (OD 600 10), at 0.2M Na 2 HPO 4 In citrate buffer, the pH is 5.4. The catalytic process was carried out at 50°C for 12 hours. GABA production as Figure 1b shown. S. cerevisiae BJ5464 / SsGAD and S. cerevisiae BJ5464 / ScGAD produced 74.8-fold and 19.5-fold higher GABA production than wild type, respectively, confirming the functional expression of these two enzymes in S. cerevisiae. The catalytic efficiency of Saccharomyces cerevisiae BJ5464 / SsGAD was significantly higher than that of BJ5464 / ScGAD, which was consistent with the results observed in ...
experiment example 3
[0055] Experimental Example 3: Comparison of GABA conversion efficiency between Saccharomyces cerevisiae BJ 5464 / SsGAD cells and SsGAD cells in different culture periods
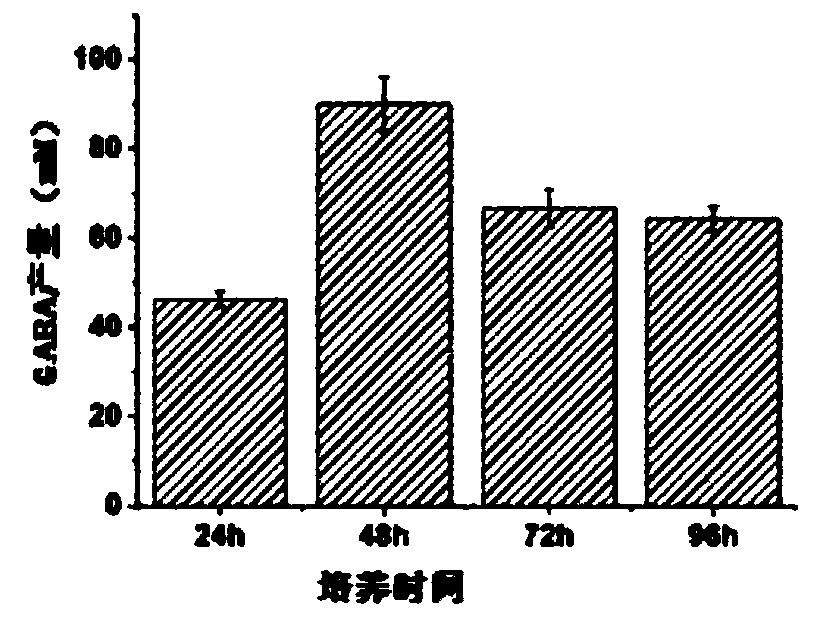
[0056]The effect on decarboxylation efficiency of Saccharomyces cerevisiae BJ5464 / SsGAD was evaluated by incubation time. Using 0.1M L-GLU as substrate, in 0.2mM Na 2 HPO 4 0.2 mM PLP was added to citrate buffer (pH 5.4). Cell density is set to OD 600 10. The reaction was carried out at 50 °C for 12 h. Such as Figure 2a As shown, the production of GABA increased from 46.3mM to 89.9mM when the culture time of Saccharomyces cerevisiae BJ5464 / SsGAD cells was extended from 24 hours to 48 hours. However, further prolonging the culture time significantly reduced the transformation ability of S. cerevisiae BJ5464 / SsGAD. When cultured for 96h compared with cultured for 48h, the production of GABA decreased by 28.6%. The GABA metabolic pathway consists of three yeasts (including GAD, GABA aminotransferase (C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com