Venom protein gel and preparation method thereof
A technology of snake venom and gel, which is applied in the fields of peptide/protein components, anti-inflammatory agents, pharmaceutical formulas, etc. It can solve the problems of snake venom that have not been seen yet, and achieve the effect of improving drug efficacy and thickening effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] 1. Preparation of isolated skin
[0039] Male SD rats with a body weight of about (200±20) g were taken, anesthetized with pentobarbital, and executed by neck dislocation. The abdomen of the rats was depilated with scissors, razors, and depilatory cream, and the skin was washed with warm water. Wrap the index finger with gauze, dip it in saline, and gently remove the abdominal fat. The removed skin was rinsed with physiological saline, wrapped in plastic wrap and tin foil, stored in a -20°C refrigerator, and used within three days.
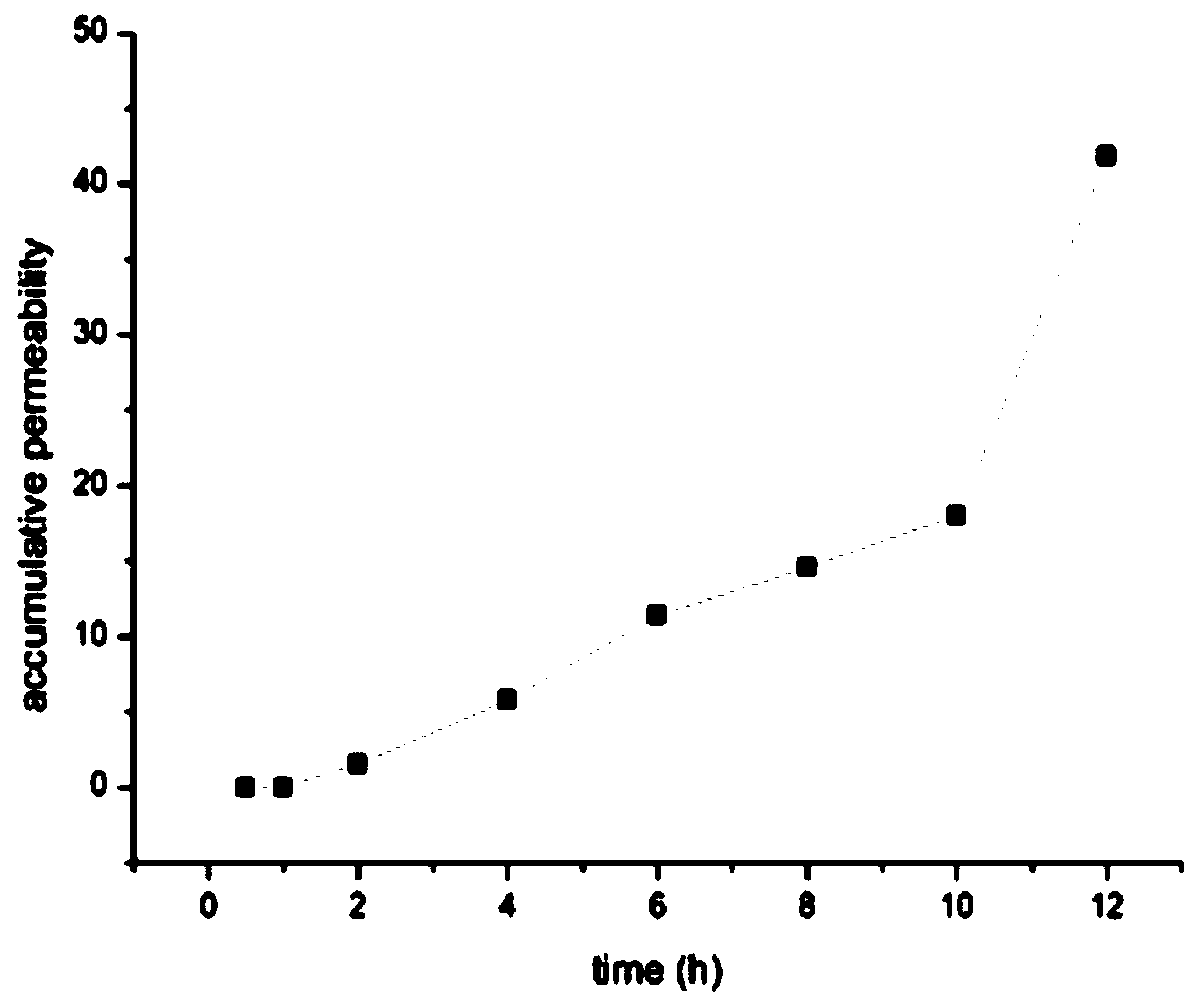
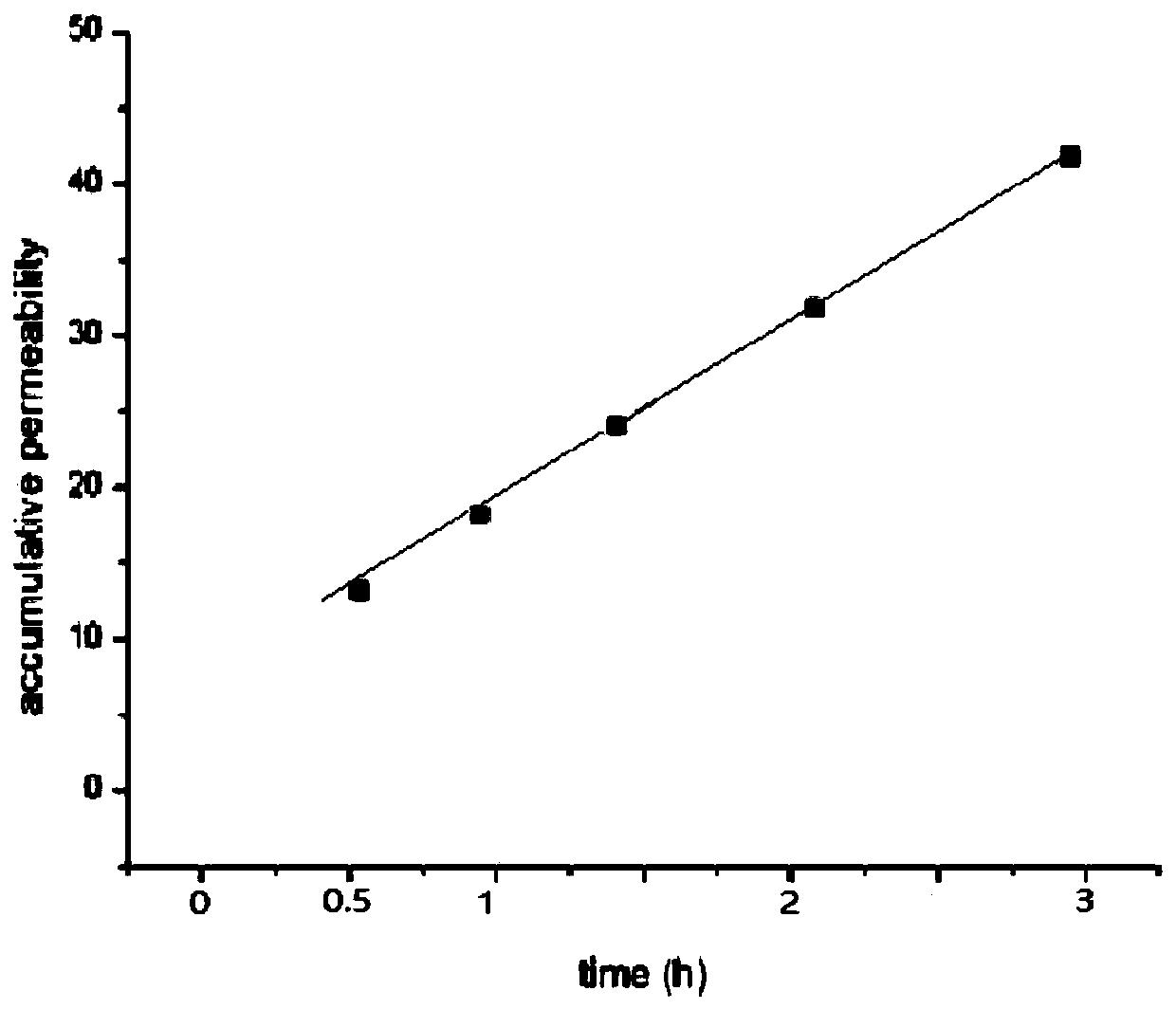
[0040] Transdermal diffusion device and its experiment: use the modified Franz diffusion cell (the upper cell is a cylindrical release cell, and the lower cell is a conical receiving cell), put the two cells together, put the skin in the middle and fix it, the skin penetration area is 2.56 cm -2 , add a small amount of snake venom solution dissolved in ultrapure water on the skin. There is a sampling tube connected to the bottom of the r...
Embodiment 1
[0053] The snake venom gel of this embodiment includes snake venom dry powder, gel matrix, transdermal absorption accelerator, protein protectant, pH regulator, solvent and other pharmaceutically acceptable auxiliary materials;
[0054] The gel matrix is carbomer 940;
[0055] The transdermal absorption accelerator is essential oil;
[0056] The protein protectant is mannitol;
[0057] The pH regulator is a compound of triethanolamine and sodium bicarbonate.
[0058] In terms of mass volume concentration, the concentration of the carbomer 940 is 0.002g / mL;
[0059] In terms of mass volume concentration, the concentration of the triethanolamine is 0.05 g / mL.
[0060] Described essential oil is peppermint oil or turpentine;
[0061] Other pharmaceutically acceptable adjuvants include:
[0062] Moisturizing agent: the moisturizing agent is glycerin;
[0063] Preservative: the preservative is ethylparaben;
[0064] The solvent is distilled water.
[0065] Snake venom gel...
Embodiment 2
[0073] The snake venom gel of this embodiment includes snake venom dry powder, gel matrix, transdermal absorption accelerator, protein protectant, pH regulator, solvent and other pharmaceutically acceptable auxiliary materials;
[0074] The gel matrix is carbomer 940;
[0075] The transdermal absorption accelerator is essential oil;
[0076] The protein protectant is mannitol;
[0077] The pH regulator is a compound of triethanolamine and sodium bicarbonate.
[0078] In terms of mass volume concentration, the concentration of the carbomer 940 is 0.0035g / mL;
[0079] In terms of mass volume concentration, the concentration of the triethanolamine is 0.046g / mL.
[0080] Described essential oil is peppermint oil or turpentine;
[0081] Other pharmaceutically acceptable adjuvants include:
[0082] Moisturizing agent: the moisturizing agent is glycerin;
[0083] Preservative: the preservative is ethylparaben;
[0084] The solvent is distilled water.
[0085] Snake venom ge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com