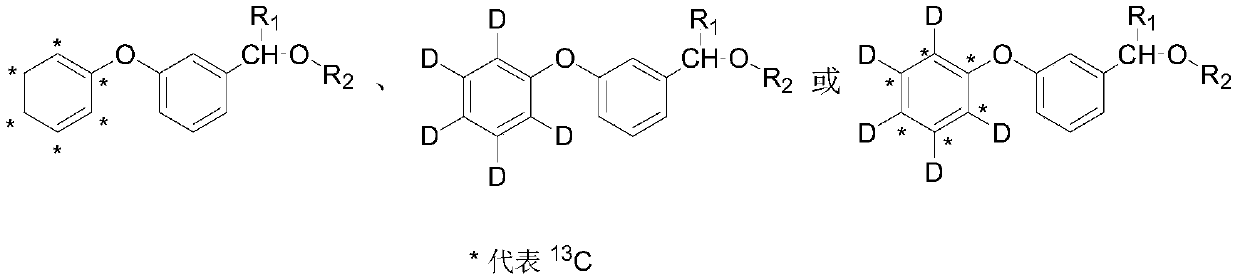
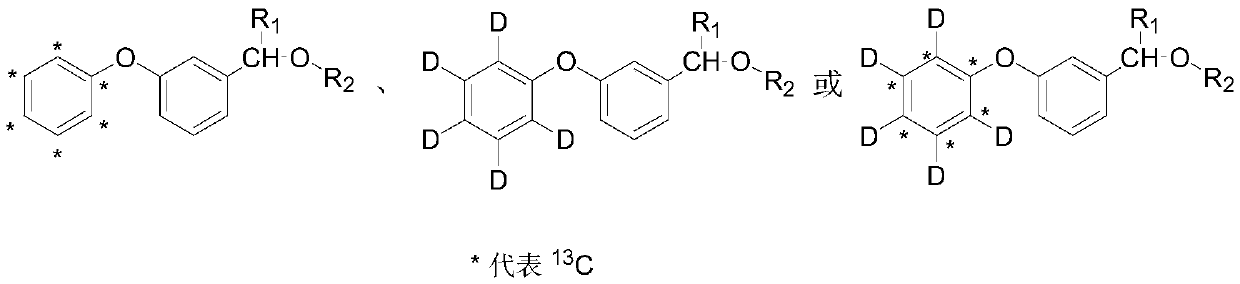
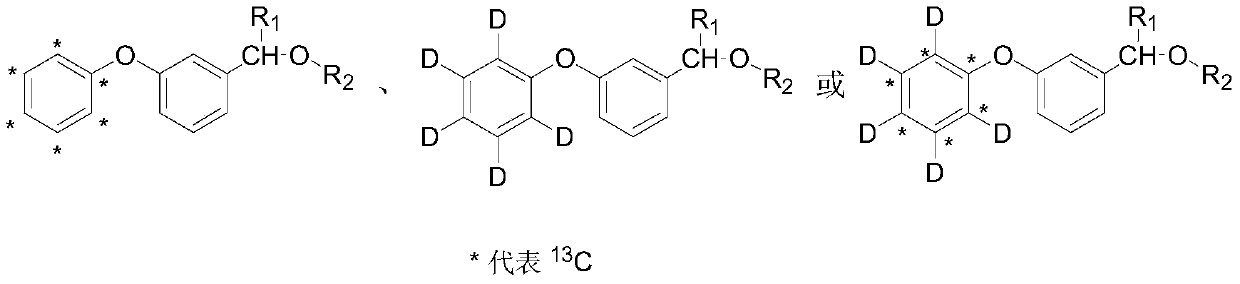
Synthesis method of stable isotope labeled pyrethroid
A stable isotope, pyrethroid technology, applied in organic chemistry methods, chemical instruments and methods, preparation of carbon-based compounds, etc., can solve the problem that raw materials are difficult to obtain, the natural abundance of m-phenoxybenzaldehyde requires a large amount, and it is easy to cause Abundance dilution and other issues to achieve the effect of reducing the generation of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A synthetic method for stable isotope-labeled pyrethroids provided by the invention comprises the following steps:
[0029] S1) Potassium phenate labeled with stable isotope is used as a labeling precursor, which reacts with m-bromobenzaldehyde under microwave conditions to generate an intermediate stable isotope-labeled m-phenoxybenzaldehyde; the stable isotope-labeled potassium phenate is a stable isotope 13 C label, stable isotope D label or stable isotope 13 C and D are double-labeled; the substituted chrysanthemum acid chloride is dichloro chrysanthemum acid chloride, dibromochrysanthemum acid chloride, trifluoromethyl chrysanthemum acid chloride, 2,2,3,3-tetramethylcyclopropanecarbonyl chloride or isopropyl p-chloro Phenylacetyl. In the step S1, the molar ratio of stable isotope-labeled potassium phenate to m-bromobenzaldehyde is 1:1-1:10, the reaction temperature is -50-200° C., and the reaction time is 0.1-2 hours. Exceeding the range will cause a huge waste o...
Embodiment 1
[0040] A stable isotope labeled cypermethrin-D 5 The preparation method, this method comprises the following steps:
[0041] 1. m-phenoxybenzaldehyde-D 5 Synthesis
[0042] Add potassium phenate-D to the 80mL reaction bottle 5 2.7g, activated cuprous chloride 1g, add m-bromobenzaldehyde 4g, DMSO (dimethyl sulfoxide) 10ml, helium protection, closed system, set microwave power 50 watts, pressure 50psi, reaction temperature 140 ℃, The reaction time is 5min. Column chromatography separation after reaction finishes, obtains 3.71g m-phenoxybenzaldehyde-D 5 , Yield 92.5%, GC detection, purity 99.5%, mass spectrometry detection, isotope abundance 99.5atom%D.
[0043] 2. Cypermethrin-D 5 Synthesis
[0044] In a 35mL reaction bottle, sequentially add 4g of sodium cyanide, 3g of heavy water, and m-phenoxybenzaldehyde-D 5 1.75g, add chrysanthemum acid chloride 4g, close the system, set the microwave power to 40 watts, the pressure to 40psi, the reaction temperature to 40°C, and t...
Embodiment 2
[0046] A stable isotope labeled permethrin- 13 C 6 The preparation method, this method comprises the following steps:
[0047] 1. m-phenoxybenzaldehyde- 13 C 6 Synthesis
[0048] Add potassium phenate- 13 C 6 5.5g, activated cuprous bromide 1.5g, add m-bromobenzaldehyde 8.1g, dioxane 15ml, argon protection, closed system, set microwave power 100 watts, pressure 80psi, reaction temperature 150 ℃, reaction time 4min. Column chromatography separation after reaction finishes, obtains 7.56g m-phenoxybenzaldehyde- 13 C 6 , yield 94.3%, GC detection, purity 99.6%, mass spectrometry detection, isotope abundance 99.8atom% 13 c.
[0049] 2. Permethrin- 13 C 6 Synthesis
[0050] In a 35mL reaction flask, sequentially add 1g of sodium borohydride, 3g of deuterated methanol, and m-phenoxybenzaldehyde- 13 C 6 0.88g, stir for 5min, add chrysanthemum acid chloride 2g, close the system, set the microwave power to 70 watts, the pressure to 45psi, the reaction temperature to 60°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com