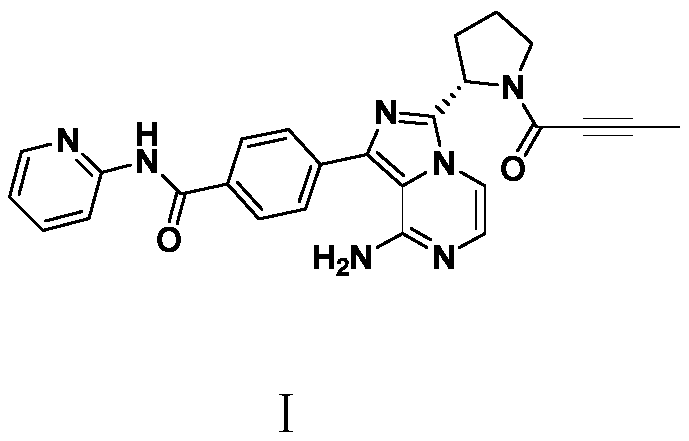
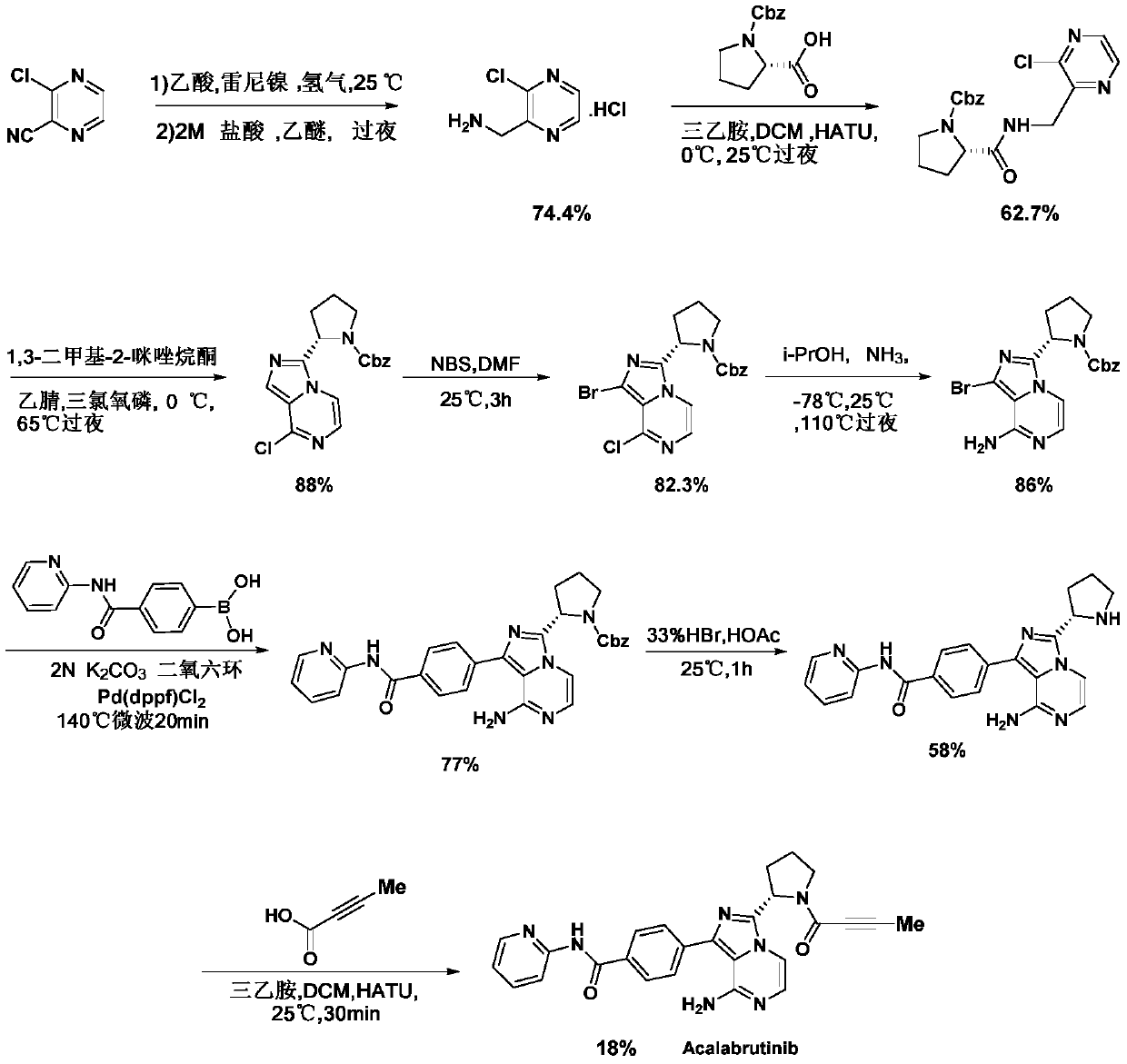
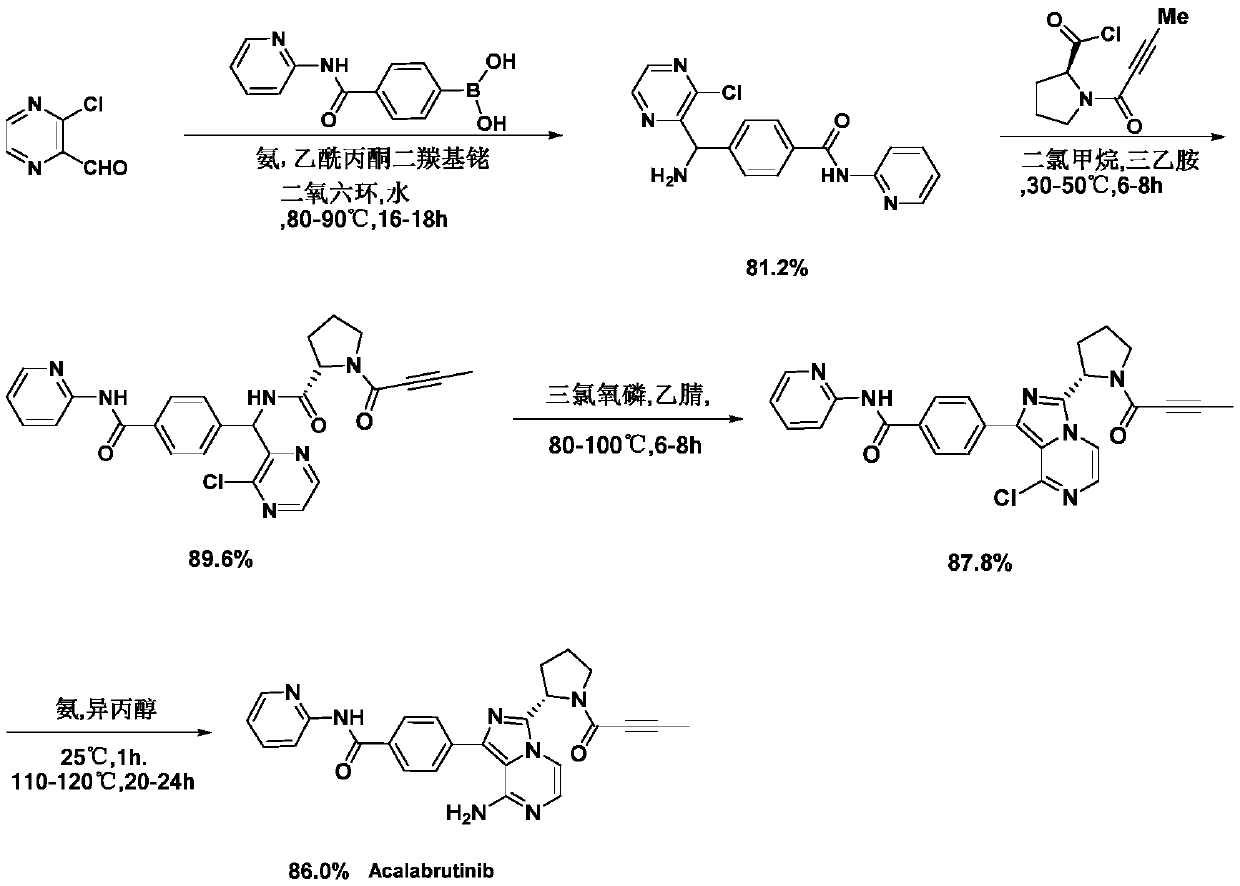
A kind of preparation method of Acotinib
A technology of acotinib and a compound, applied in the field of preparation of acotinib, can solve the problems of difficult industrial operation, difficult purification, difficult to obtain, etc., and achieves a simple and efficient separation and purification method, short process steps, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of N-(pyridin-2-yl)-4-cyanoacetylbenzamide (Ⅲ)
[0065] To a 500 ml four-neck flask connected with stirring, a thermometer, a reflux condenser and a dropping funnel, add 150 grams of tetrahydrofuran, 6.0 grams of acetonitrile, 16.8 grams (0.15 moles) of potassium tert-butoxide, between 30 and 35 ° C, drop Add 25.6 g (0.1 mole) of a mixture of N-(pyridin-2-yl) monomethyl terephthalate monoamide and 50 g of tetrahydrofuran, drop it, and react with stirring at 35 to 40° C. for 5 hours. Cool to 20 to 25°C, acidify the pH value of the system to 3.0-4.0 with 20wt% ammonium chloride aqueous solution, add 100 grams of dichloromethane, separate layers, and extract the aqueous layer with dichloromethane 3 times, 20 grams each time, combine the organic phase, dichloromethane was recovered by distillation to obtain 23.9 g of N-(pyridin-2-yl)-4-cyanoacetylbenzamide, the yield was 90.2%, and the liquid phase purity was 99.2%.
Embodiment 2
[0066] Example 2: Preparation of N-(pyridin-2-yl)-4-cyanoacetylbenzamide (Ⅲ)
[0067] To a 500 ml four-neck flask connected with stirring, a thermometer, a reflux condenser and a dropping funnel, add 150 grams of tetrahydrofuran, 6.0 grams of acetonitrile, 16.8 grams (0.15 moles) of potassium tert-butoxide, between 40 and 45 ° C, drop Add 27.0 g (0.1 mol) of a mixture of N-(pyridin-2-yl) monoethyl terephthalate monoamide and 50 g of tetrahydrofuran, drop it, and react with stirring at 40 to 45° C. for 4 hours. Cool to 20 to 25°C, acidify the pH value of the system to 3.0-4.0 with 20wt% ammonium chloride aqueous solution, add 100 grams of dichloromethane, separate layers, and extract the aqueous layer with dichloromethane 3 times, 20 grams each time, combine the organic phase, dichloromethane was recovered by distillation to obtain 24.1 g of N-(pyridin-2-yl)-4-cyanoacetylbenzamide, the yield was 90.9%, and the liquid phase purity was 99.1%.
Embodiment 3
[0068] Example 3: Preparation of N-(pyridin-2-yl)-4-cyanoacetylbenzamide (Ⅲ)
[0069] To a 500 ml four-neck flask connected with stirring, a thermometer, a reflux condenser and a dropping funnel, add 150 grams of tetrahydrofuran, 5.5 grams of acetonitrile, 10.0 grams (0.15 moles) of sodium ethylate, between 40 and 45 ° C, dropwise add 25.6 A mixture of gram (0.1 mole) of N-(pyridin-2-yl) monomethyl terephthalate monoamide and 50 grams of tetrahydrofuran was added dropwise, and stirred at 45 to 50° C. for 4 hours. Cool to 20 to 25°C, acidify the pH value of the system to 3.0-4.0 with 20wt% ammonium chloride aqueous solution, add 100 grams of dichloromethane, separate layers, and extract the aqueous layer with dichloromethane 3 times, 20 grams each time, combine the organic phase, dichloromethane was recovered by distillation to obtain 23.6 g of N-(pyridin-2-yl)-4-cyanoacetylbenzamide, the yield was 89.1%, and the liquid phase purity was 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com