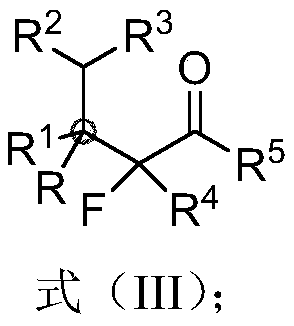
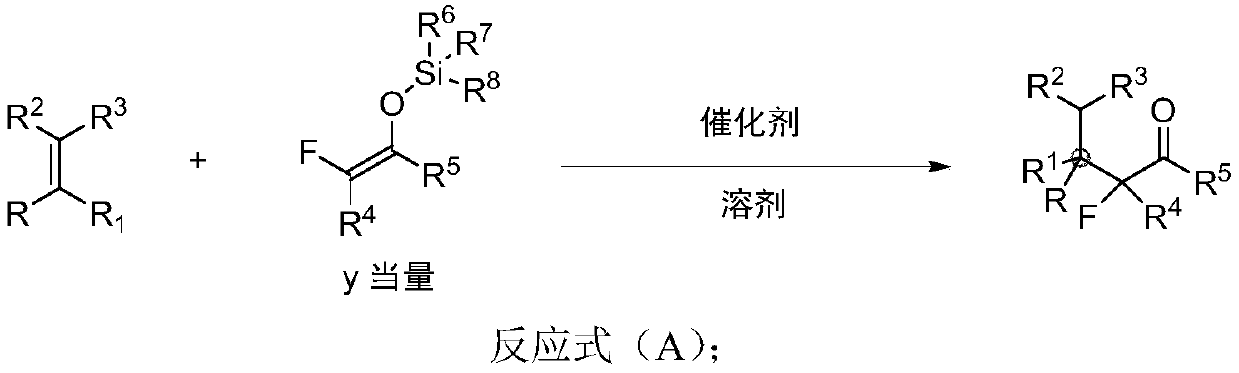
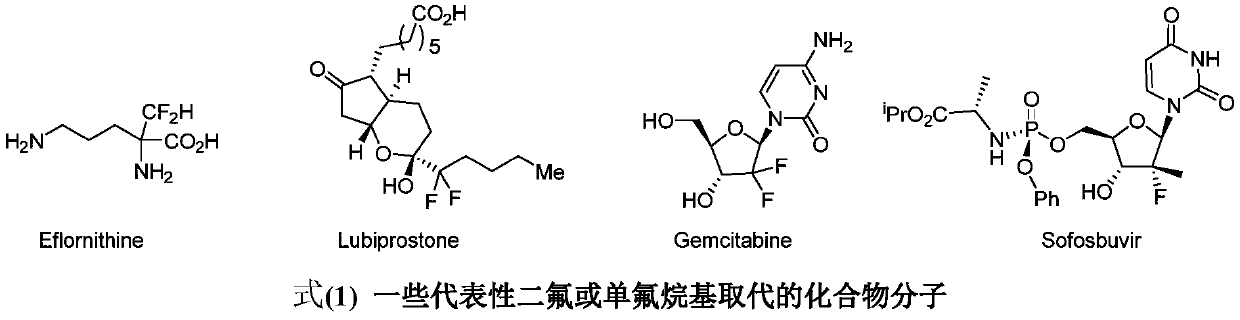
Beta-all carbon quaternary carbon-alpha-fluoroalkyl ketone compound and synthesis method and application thereof
A technology of fluoroalkyl ketones and synthetic methods, which is applied in the fields of carbon-based compound preparation, organic chemical methods, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Synthesis of β-all-carbon quaternary carbon-α-fluoroalkyl ketone Ⅲ-1:
[0086]
[0087] Add 3.6 mg perchloric acid (70% wt), distilled water (2.5 mL), olefin I-1 (33.1 mg, 0.25 mmol), and phenyl-substituted difluoroenol silyl ether II-1 to a 5.0 mL reaction flask in sequence (570mg, 2.5mmol), the reaction solution was stirred at 50°C for 15h. TLC detects that the raw materials have basically reacted, and the reaction is stopped. The reaction solution was extracted with ethyl acetate for 3 times, dried over anhydrous sodium sulfate, transferred to a 50mL round bottom flask, added about 1.0g of crude silica gel, spin-dried, followed by column chromatography and eluent (petroleum ether), to obtain product III- 1 is 66.3mg of colorless liquid, the yield is 92%. 1 H NMR (400MHz, CDCl 3 ):δ7.56-7.54(m,2H),7.45-7.41(m,1H),7.36-7.34(m,2H),7.26-7.17(m,5H),2.46-2.37(m,1H),1.99 -1.90(m,1H),1.60(s,3H),0.75(t,J=7.2Hz,3H); 13 C NMR (100MHz, CDCl 3 ): δ191.28(t, J=30.3Hz), 13...
Embodiment 2
[0089] Synthesis of β-all-carbon quaternary carbon-α-fluoroalkyl ketone Ⅲ-2:
[0090]
[0091] Add trifluoromethanesulfonic acid (3.8mg, 0.025mmol), olefin I-2 (37.6mg, 0.25mmol), phenyl-substituted difluoroenolsilyl ether II-1 (85.5, 0.375 mmol), tetrahydrofuran (2.5 mL); the reaction solution was stirred at room temperature for 5 h. TLC detects that the raw material has basically reacted completely, and the reaction is stopped. The reaction solution was transferred to a 50mL round-bottomed flask, and the solvent was drained under the oil pump, followed by direct column chromatography and eluent (DCM / petroleum ether=1 / 20) to obtain 63.6 mg of product III-2 as a colorless liquid, Yield 83%. 1 H NMR (400MHz, CDCl 3 ):δ7.64-7.62(m,2H),7.49-7.46(m,1H),7.33-7.26(m,4H),6.93-6.89(m,2H),2.40-2.31(m,1H),1.98 -1.89(m,1H),1.58(s,3H),0.73(t,J=1.9Hz,3H); 13 C NMR (100MHz, CDCl 3 ): δ190.93(t, J=30.2Hz), 161.88(d, J=245.3Hz), 134.22(t, J=1.9Hz), 133.98(dd, J=4.8, 4.5Hz), 133.40, 1...
Embodiment 3
[0093] Synthesis of β-all-carbon quaternary carbon-α-fluoroalkyl ketone Ⅲ-3:
[0094]
[0095] In the 5.0mL reaction flask, add H 2 SO 4 (5mg, 0.05mmol), olefin I-3 (41.7mg, 0.25mmol), phenyl-substituted difluoroenol silyl ether II-1 (285.4mg, 1.25mmol), toluene (2.5mL); After stirring at 30°C for 20h. TLC detects that the raw material has basically reacted completely, and the reaction is stopped. Transfer the reaction solution to a 50mL round-bottomed flask, drain the solvent under the oil pump, perform direct column chromatography, eluent (dichloromethane / petroleum ether=1 / 20), and obtain product III-3 as a colorless liquid 68.4 mg, yield 85%. 1 H NMR (400MHz, CDCl 3 ):δ7.67-7.65(m,2H),7.49-7.46(m,1H),7.31-7.24(m,4H),7.20-7.18(m,2H),2.38-2.29(m,1H),1.97 -1.88(m,1H),1.56(s,3H),0.72(t,J=7.6Hz,3H); 13 C NMR (100MHz, CDCl 3 ): δ190.60(t, J=30.3Hz), 136.91(t, J=2.4Hz), 134.11(t, J=2.1Hz), 133.46, 133.22, 130.18, 129.77(t, J=4.1Hz), 128.16, 128.11, 120.58(t, J=260.2Hz)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com