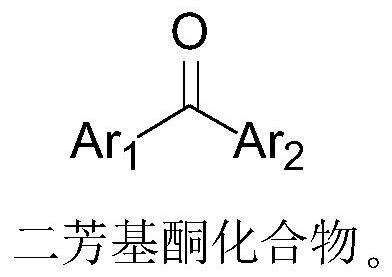
A kind of method of one-pot synthetic diaryl ketal
A synthesis method and dimethoxy technology, which are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as not meeting the requirements of green manufacturing, and achieve short steps, good practicability, and simple post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: the synthesis of 1-(dimethoxy-phenyl-methyl)-4-methoxy-benzene
[0025]
[0026] 250mL four-necked flask with magnetic stirring, thermometer and reflux condenser, successively added trifluoroacetic anhydride (42.0g, 200mmol), anisole (13.0g, 120mmol), boron trifluoride etherate complex (1.42g, 10mmol ), benzoic acid (12.2 g, 100 mmol) was added in portions, and the temperature was raised to 30-40° C. for 6 hours under stirring to obtain a dark brown solution. Cool down to room temperature, add methanol (60mL) and trimethyl orthoformate (31.8g, 300mmol), the color of the solution turns light red, heat up to 55-60°C for 18h, and add triethylamine (2.0g) after cooling down The pH was adjusted to alkaline, and the color of the solution further became pale yellow. Concentrate under reduced pressure to remove most of the solvent, and a solid precipitates. Add heptane (50mL) to make a slurry, filter, and air-dry at 40°C to obtain white 1-(dimethoxy-phenyl-m...
Embodiment 2
[0027] Embodiment two: the synthesis of 1-(dimethoxy-phenyl-methyl)-4-isopropyl-benzene
[0028]
[0029] 250mL four-necked flask with magnetic stirring, thermometer and reflux condenser, successively added trifluoroacetic anhydride (42.0g, 200mmol), cumene (14.4g, 120mmol), boron trifluoride diethyl ether complex (1.42g, 10mmol ), benzoic acid (12.2g, 100mmol) was added in portions, and the temperature was raised to 30-40° C. for 4 hours under stirring to obtain a wine-red solution. Cool down to room temperature, add methanol (60mL) and trimethyl orthoformate (31.8g, 300mmol), the color becomes light yellow, heat up to 55-60°C for 18h, add triethylamine (2.0g) to adjust the temperature pH to alkaline, the color of the solution further becomes lighter. Concentrate under reduced pressure to remove most of the solvent, add dichloromethane (60mL) to dissolve, wash twice with water (30mL*2), collect the organic layer, concentrate under reduced pressure to obtain an oily substa...
Embodiment 3
[0030] Embodiment three: the synthesis of 2-chloro-5-[dimethoxy-(4-methoxy-phenyl)-methyl]-thiophene
[0031]
[0032] 250mL four-necked flask with magnetic stirring, thermometer and reflux condenser, successively added trifluoroacetic anhydride (42.0g, 200mmol), anisole (16.2g, 150mmol), boron trifluoride diethyl ether complex (2.13g, 15mmol ), 5-chloro-2-thiophenecarboxylic acid (16.3 g, 100 mmol) was added in portions, and the temperature was raised to 30-40° C. for 5 hours under stirring to obtain a deep reddish-brown solution. Cool down to room temperature, add methanol (60 mL) and trimethyl orthoformate (31.8 g, 300 mmol), the color change is not obvious. Reheat to 55-60°C and keep it warm for 24 hours. After cooling down, add triethylamine (3.0 g) to adjust the pH to alkaline, concentrate most of the solvent to obtain a reddish-brown oil, add dichloromethane (80 mL) to dissolve, and water ( 40mL*2) washed twice, collected the organic layer, concentrated under reduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com