Synthetic method for poly(3-hydroxybutyrate) oligomers, product obtained by method and application of oligomers
A synthetic method, the technology of hydroxybutyric acid, applied in the direction of active ingredients of esters, botanical equipment and methods, chemicals for biological control, etc., can solve the problems of high purification and process costs, long production cycle, etc., and achieve stability Long-lasting antibacterial activity, shortened reaction time, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
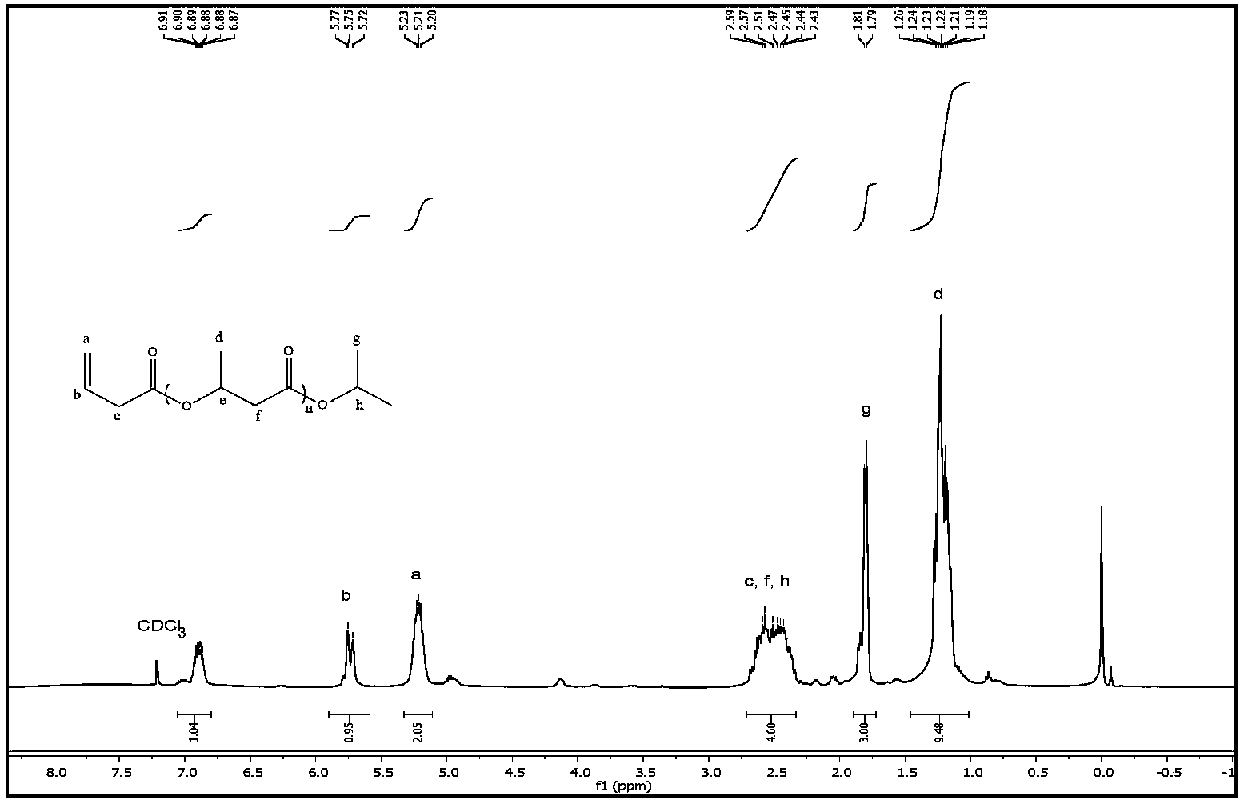
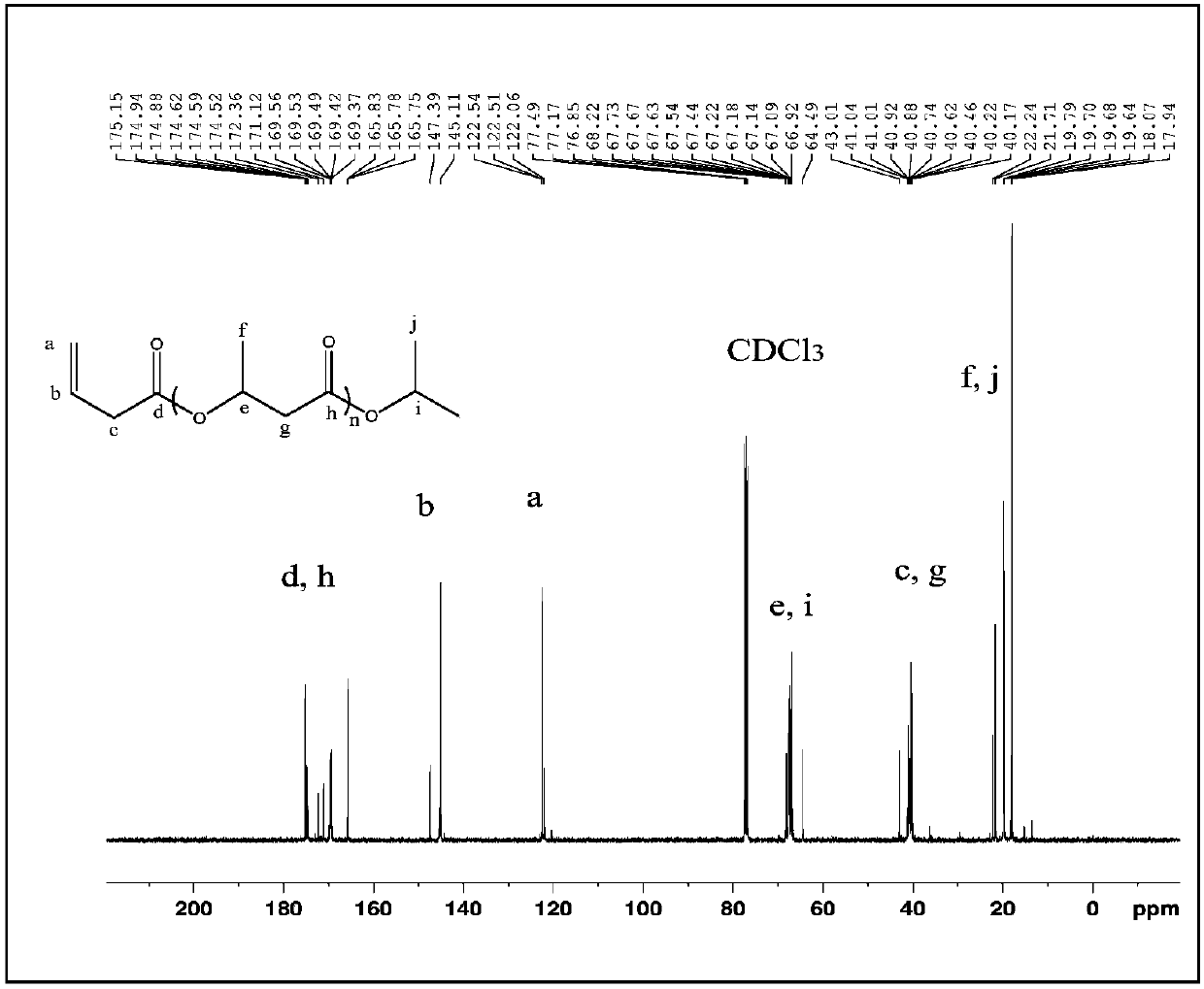
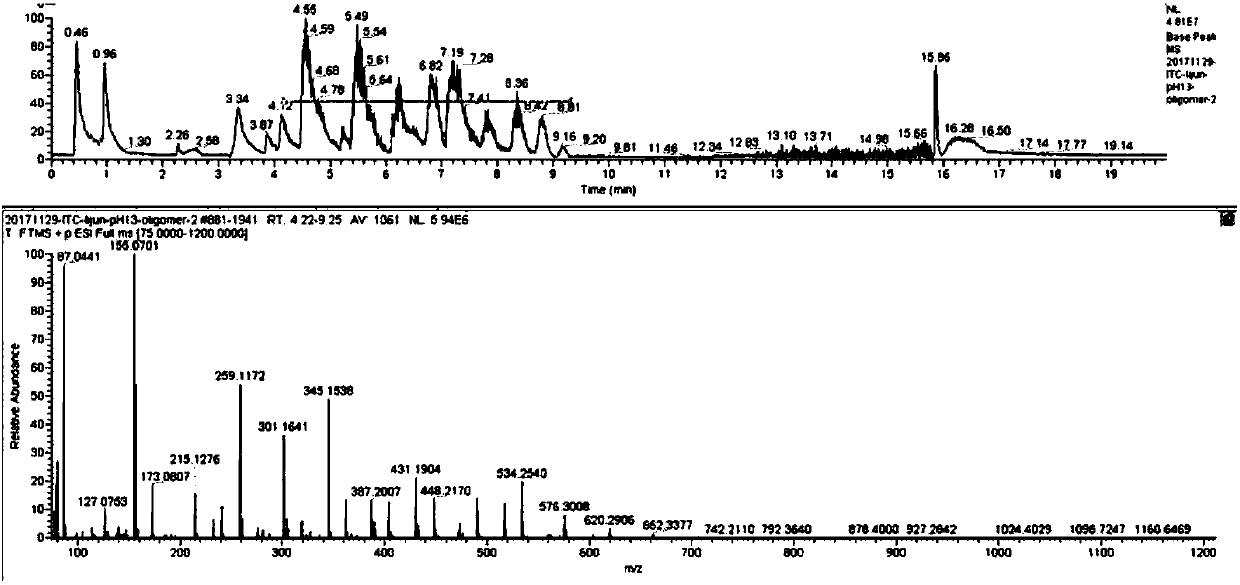
[0046]Add 0.61g (3mmol) of aluminum isopropoxide, 2.5mL of pyridine and 5.2g (60mmol) of β-butyrolactone into the reactor, and react at 50°C under nitrogen protection. After reacting for about 15 minutes, the reaction solution was bright yellow, turned green after a while, then gradually deepened, and turned brown after 24 hours of reaction. After the reaction was completed and cooled to room temperature, the reaction solution was added to 20 mL of 2M dilute hydrochloric acid, stirred for a period of time and then extracted three times with 20 mL of dichloromethane. 1) and deionized water (20 mL×2) to wash the organic layer. After drying over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure to obtain a yellow oil. The obtained oil was purified by rapid flushing with dichloromethane, the solvent was distilled off under reduced pressure, and then dried in vacuo at 50°C overnight to obtain 4.0 g of light yellow oil with a yield of 77%.
[0047] Tak...
experiment example
[0054] The oligomeric 3-hydroxybutyrate prepared in the examples was diluted to 20 mg / mL with phosphate buffered saline (PBS), and its antibacterial activity was detected by the shaking method (with reference to the national standard method GB-T20944.3-2008), and the PBS group Do a blank control. The obtained antibacterial performance results are shown in Table 1.
[0055] Table 1
[0056]
[0057] As can be seen from the results in Table 1: the oligomeric 3-hydroxybutyrate prepared by the synthetic method of the present invention has an efficient antibacterial effect on various bacteria and fungi such as Staphylococcus aureus, Klebsiella pneumoniae, and Candida albicans, and the antibacterial effect can reach >99.99%, but also has a broad-spectrum antibacterial effect.
[0058] The above experiment was repeated several times with the oligomeric 3-hydroxybutyrate, and the results of each experiment showed an antibacterial activity greater than 99.99%. After being placed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com