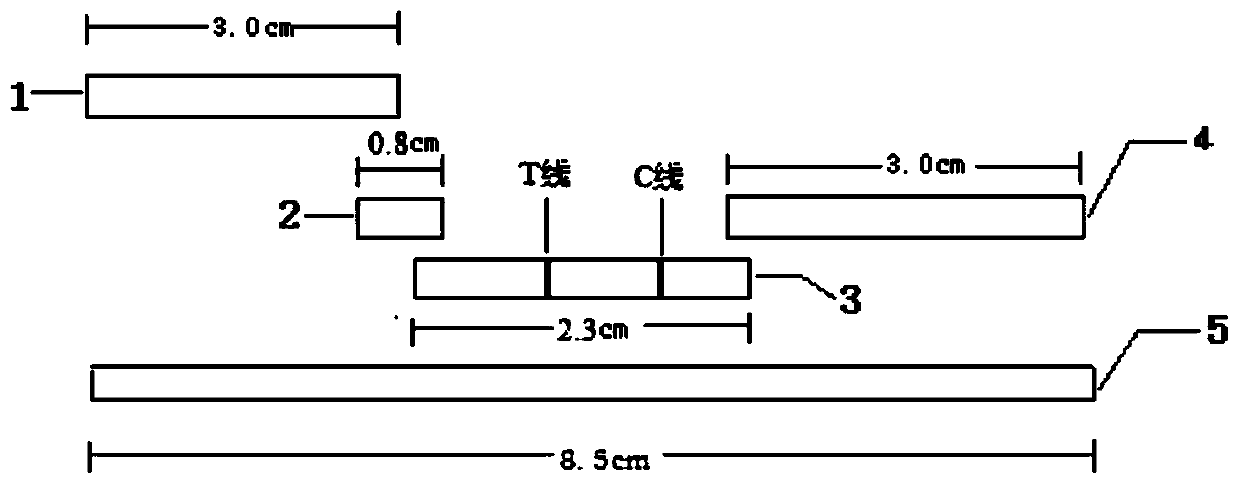
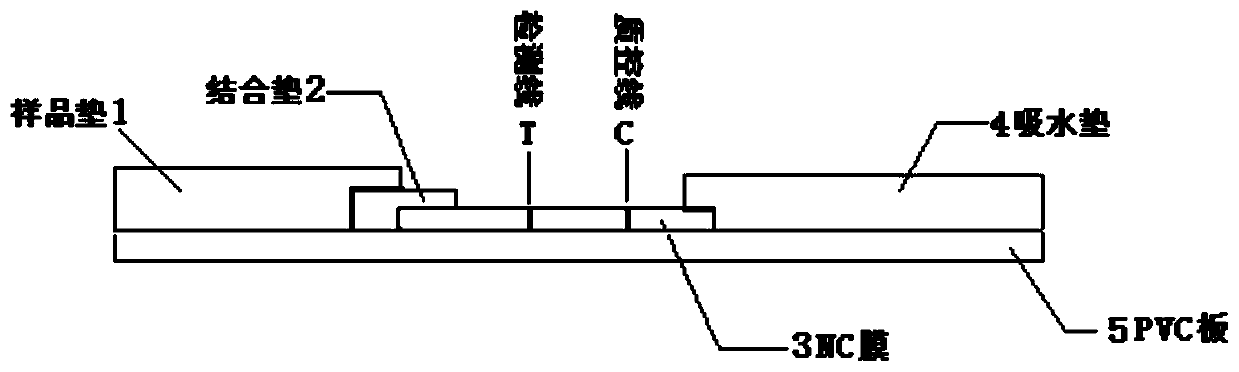
Preparation method of latex microsphere immunochromatography test paper based on moraxella catarrhalis surface protein
A technology of surface protein and latex microspheres, applied in the direction of anti-bacterial immunoglobulin, immunoglobulin, chemical instruments and methods, etc., can solve problems such as difficult to meet rapid identification, affect the effect of amplification, and not suitable, and achieve The preparation cost is low, the shelf life is extended, and the effect of strong antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of Antibody to Moraxella catarrhalis Surface Protein (M35+McaP):
[0061] 1.1) Cloning of Moraxella catarrhalis M35mac fusion gene
[0062] Obtain the peptides with the most abundant antigenic epitopes in the extracellular domain of the surface protein M35 and McaP of Moraxella catarrhalis (the accession numbers in the NCBI protein database are AY905613 and EF075940 respectively), find their gene coding sequences, and optimize their gene coding sequences , and link the two sequences with the coding sequence of the flexible linker peptide (ggsggsggsggs) to form a fusion gene. At the same time, the entire gene sequence was chemically synthesized after introducing the restriction site NdeI at the 5' end of the fusion gene, the termination signal TAA and the restriction site BamHI at the 3' end, and recorded as m35mac. The full sequence of the gene and the encoded amino acid sequence are shown in the sequence table. Specifically, the protein sequence encoded b...
Embodiment 2
[0091] Preparation of Moraxella catarrhalis surface protein (OlpA+OmpCD) antibody:
[0092] 2.1) Cloning of Moraxella catarrhalis Olpomp fusion gene
[0093] Obtain the peptides with the most abundant antigenic epitopes in the extracellular domain of the surface proteins OlpA and OmpCD of Moraxella catarrhalis (the accession numbers in the NCBI protein database are DQ996463 and AAA66180 respectively), find their gene coding sequences, and optimize their gene coding sequences , and the two sequences are connected with the coding sequence of the rigid linker peptide (eaaakeaaak) to form a fusion gene. At the same time, the entire gene sequence was chemically synthesized after introducing the restriction site NdeI at the 5' end of the fusion gene, the termination signal TAA and the restriction site BamHI at the 3' end, which was denoted as Olpomp. The full sequence of the gene and the encoded amino acid sequence are shown in the sequence table. Specifically, the protein sequenc...
Embodiment 3
[0123] Preparation of latex microsphere markers for antibodies against Moraxella catarrhalis surface protein (M35+McaP):
[0124] 3.1) Activation of latex microspheres
[0125] Take 1 mL of a solution of red carboxylated polystyrene latex microspheres (100 nm) with a concentration of 10%, and add 9 mL of 2-(N-morpholino)ethanesulfonic acid (MES) buffer solution (0.1mol / L MES, pH8.5 ) and mix well; use MES buffer to prepare 10mg / mL of N-hydroxysuccinimide (NHS) and 10mg / mL of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt salt (EDC) solution;
[0126] Add 1mL NHS solution and 1mL EDC solution to the polystyrene latex microsphere (100nm) solution in turn, mix slowly at room temperature for 30 minutes, centrifuge at 19000g for 20 minutes after incubation, remove the supernatant, and use 10mL borax buffer ( 0.1mol / L Na 2 B 4 o 7 , pH8.5) resuspension, oscillation, ultrasonic treatment (ultrasonic breaker product model: YJ92-IIDN, power 50W, working time 2s, interval time ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com