Preparation method and application of tridentate isonitrile and metal-organic micro-porous framework material MOMFs
A technology of organometallic and framework materials, applied in organic substitution, organic chemistry, organic compound/hydride/coordination complex catalysts, etc., can solve problems such as difficult recycling, low catalytic activity, and complicated preparation methods, and achieve catalyst The effect of low loss, short reaction time and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0066] A typical implementation of the present disclosure provides a tridentate carboxamide organic ligand, whose chemical structural formula is:
[0067]
[0068] Another embodiment of the present disclosure provides a method for preparing the above-mentioned organic ligand, which is obtained by sonogashira coupling reaction of 2,6-diisopropyl-p-iodobenzamide and 1.3.5 triynylbenzene.
[0069] In one or more examples of this embodiment, the sonogashira coupling reaction conditions are: 2,6-diisopropyl p-iodobenzamide and 1.3.5 triynylbenzene in Pd(pph2)Cl and CuI catalyst Under catalysis, tetrahydrofuran was added as solvent to DIEA and reacted at 50°C for 14 hours.
[0070] The third embodiment of the present disclosure provides monomers for preparing MOMFs, the chemical structural formula of which is:
[0071]
[0072] The fourth embodiment of the present disclosure provides a method for preparing the above-mentioned monomer, which is prepared by adding tridentate fo...
Embodiment 1
[0092] Example 1: Preparation of tridentate benzamide organic ligand (A).
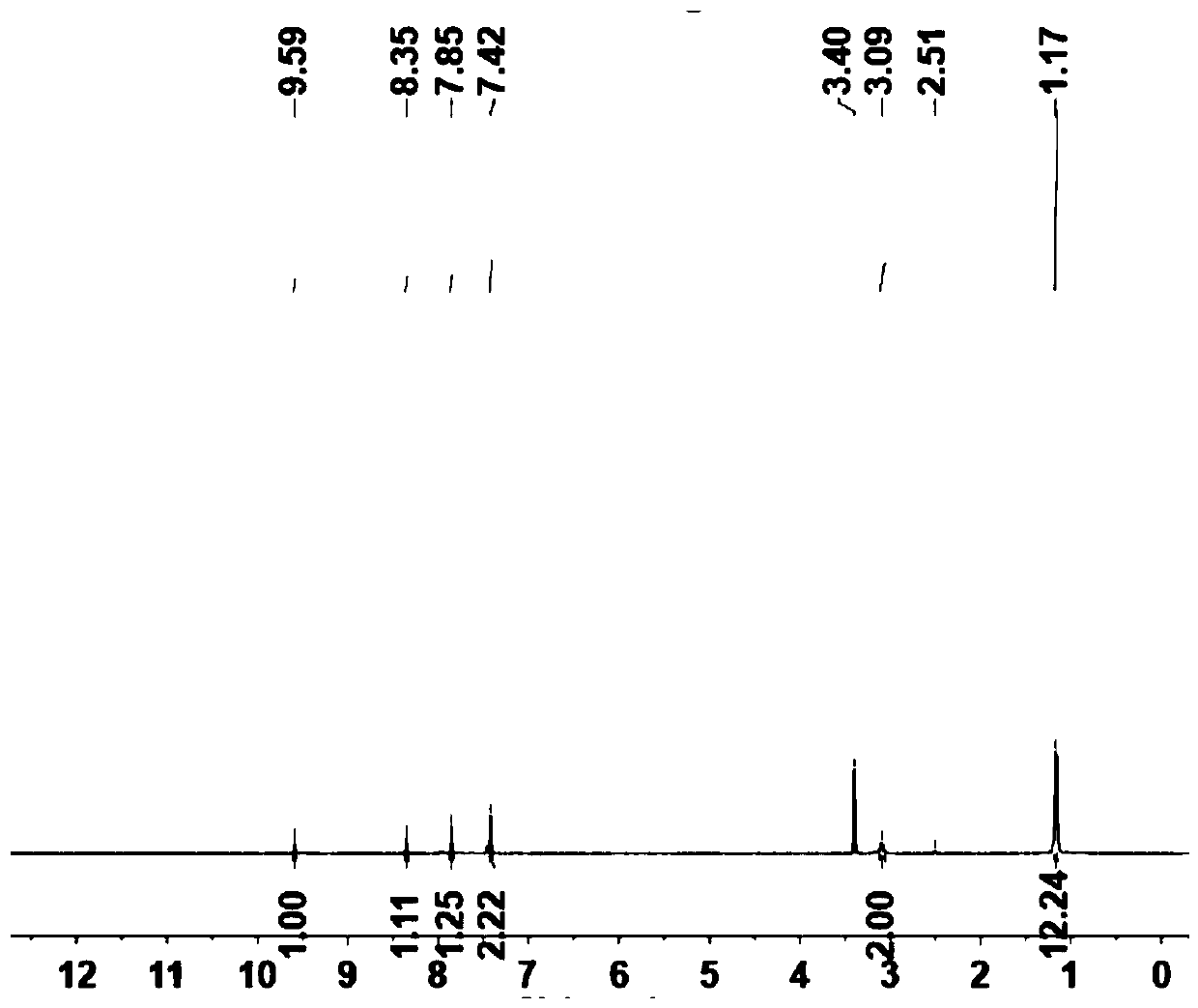
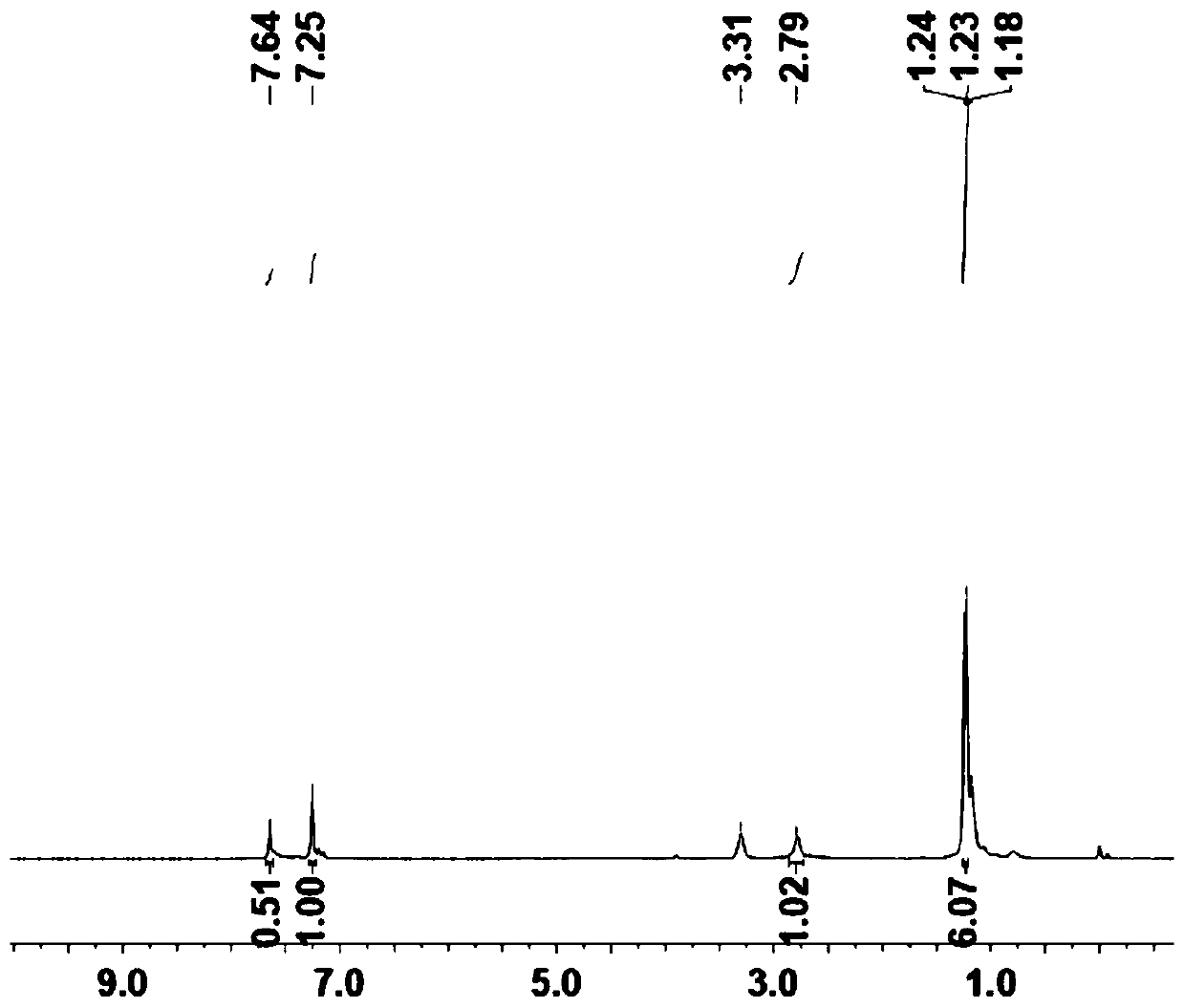
[0093] (1) Under nitrogen atmosphere, add 2.6 diisopropyl p-iodobenzamide (3mmol, 0.994g) and 1.3.5 triynylbenzene (1mmol, 0.150g), then add the catalyst [1,1'-bis (Diphenylphosphine) ferrocene] palladium dichloride (II) Pd (pph2) cl2 (0.1mmol, 75mg) and cocatalyst CuI (0.3mmol, 114mg), then add 20ml of THF and DIEA in the reaction flask (3mmol, 0.496ml), react at 50°C for 14h, cool to room temperature after the reaction, filter with suction, and wash the resulting solid with dichloromethane to remove the raw material. The product was then further purified on silica gel with the ratio of dichloromethane to methanol (50:1) as the eluent to give the pure product as a yellow solid. The yield was (0.494 g, 64%).
[0094] The structural characterization of the prepared A is as follows Figure 1~2 shown.
[0095] The synthetic route of A is as follows:
[0096]
Embodiment 2
[0097] Example 2: Preparation of monomer (B) of MOMFs.
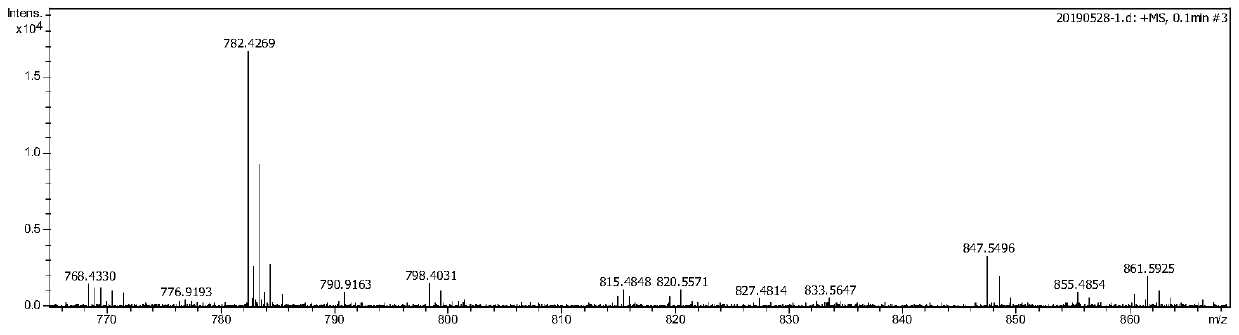
[0098] Under nitrogen, A (1 mmol, 0.759 g) and triethylamine (10 mmol, 1.011 g, 4.17 ml) were mixed in 50 mL of anhydrous dichloromethane and placed in a 100 mL Schlenk flask. After stirring for 20min at 0°C in nitrogen, add POCl to the reaction system 3 (2mmol, 0.306g, 1.96ml), then stirred at 0°C for 1h, and added 10% Na 2 CO 3 Adjust the pH value of the aqueous solution to be neutral, then extract with dichloromethane, wash with saturated sodium chloride solution, and rotary evaporate to obtain the crude product. The product is further purified with a silica gel column, and the eluent is ethyl acetate to petroleum ether at a ratio of 100:1. .Obtain B as a brick red solid (0.635g, 90.0%), and the structural characterization is as follows Figure 3-4 shown.
[0099] The synthetic route of B is as follows:
[0100]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com