Acid phosphatase detection method based on cysteamine-N-acetyl-L-cysteine-gold nanocluster fluorescent material
A gold nanocluster, acid phosphatase technology, applied in analytical chemistry and nano fields, can solve the problems of using toxic metal ions, complicated operation steps, interference, etc., and achieve the effects of strong specificity, high detection sensitivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of gold nanoclusters: Mix 0.75 mL cysteamine (30 mmol / L) and 0.25 mL N-acetyl-L-cysteine (30 mmol / L) in advance, add 8 mL ultrapure water and mix well, then Add 1 mL of chloroauric acid (20 mmol / L), incubate in a 90°C water bath for 1.5 h, take out and centrifuge to remove large particles of nanoparticles, use a dialysis bag with a molecular weight cut-off of 3500, and dialyze in double distilled water for 24 h to obtain Co-protected gold nanocluster solution with cysteamine and N-acetyl-L-cysteine. All glassware used in the preparation was soaked in aqua regia, washed thoroughly with double-distilled water, and air-dried.
Embodiment 2
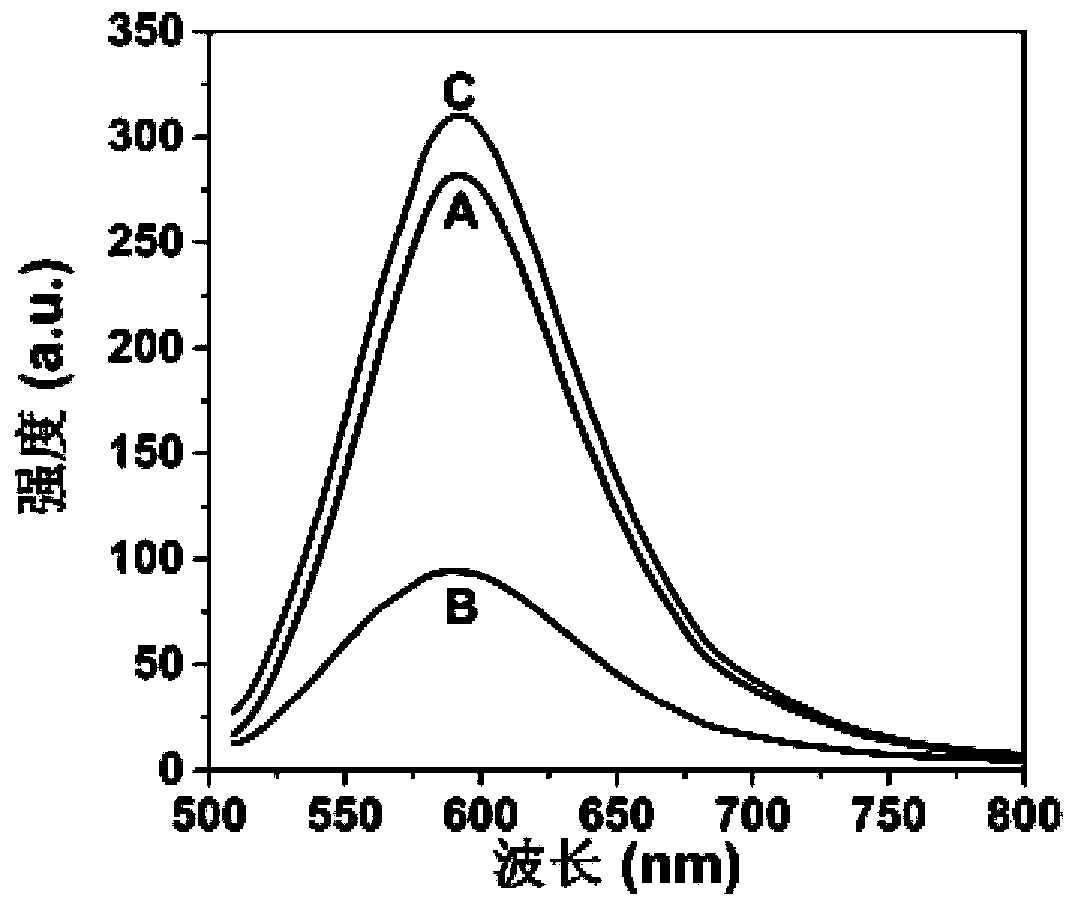
[0033] Add 0.2 mL of pyridoxal phosphate solution with a concentration of 25 μmol / L and 0.2 mL of acid phosphatase solution with a final concentration of 5 U / L into 1.45 mL of acetate buffer (pH 5.0, 50 mmol / L), After mixing, place in a 37°C water bath for 30 min. After the end, 0.15 mL of the gold nanocluster solution prepared in Example 1 was mixed with the above reaction solution, and the emission spectrum was measured immediately after mixing (excitation wavelength was 425 nm). Set up a control group. It can be seen from the figure that the gold nanocluster solution itself has obvious emission at 590nm ( figure 1 A in A); when pyridoxal phosphate was added, the fluorescence of the gold nanocluster solution was significantly inhibited ( figure 1 B in B); and when the system contains acid phosphatase, the fluorescence quenching of gold nanocluster solution caused by pyridoxal phosphate can be restored ( figure 1 in C).
Embodiment 3
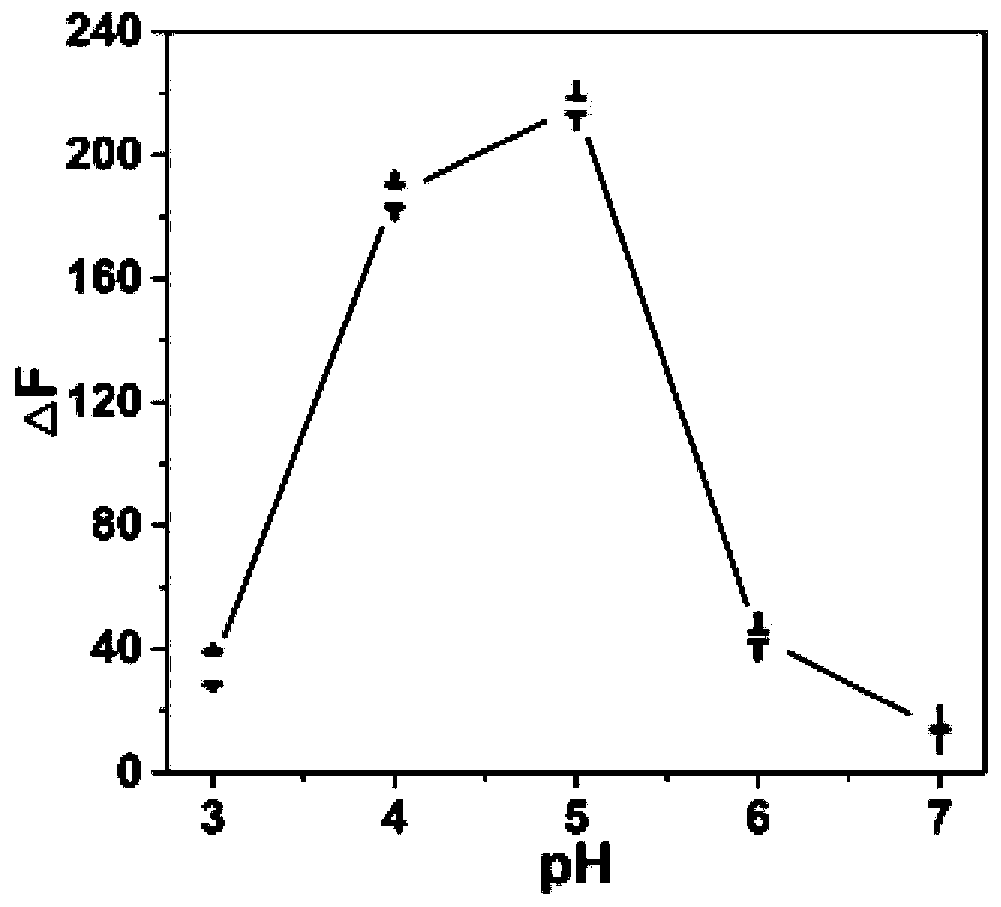
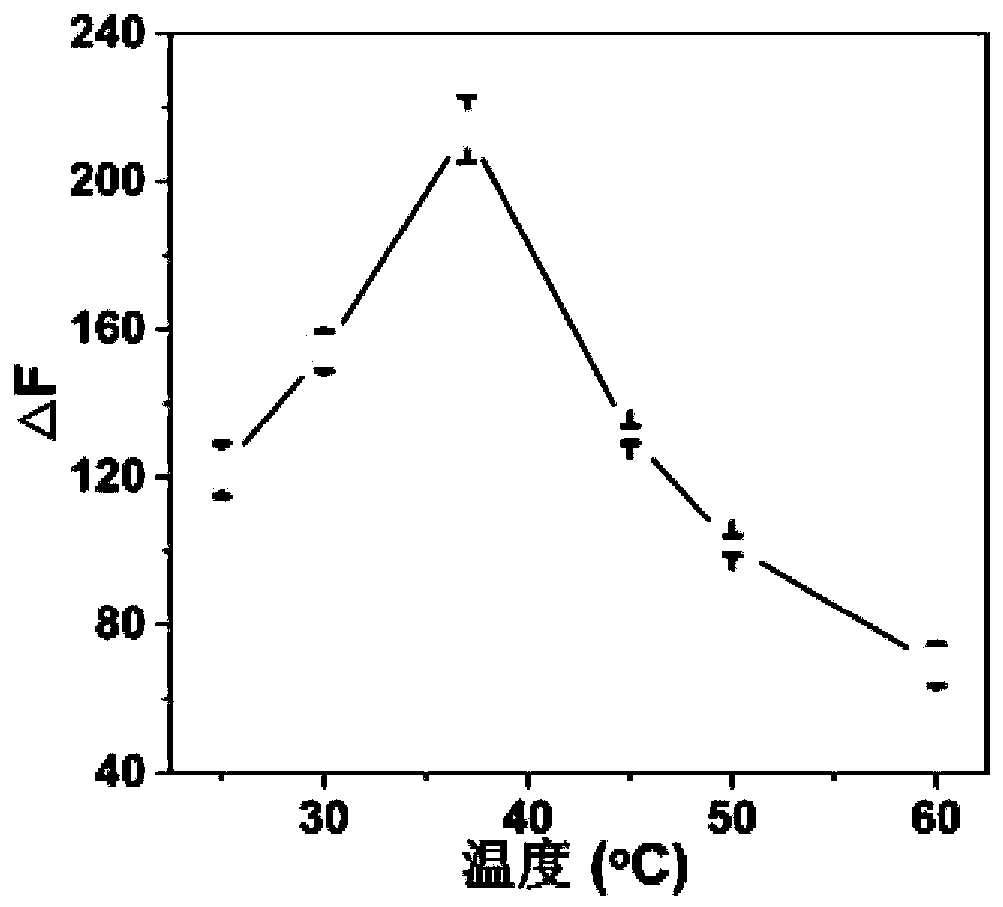
[0035] Add 0.2 mL of pyridoxal phosphate solution with a concentration of 25 μmol / L and 0.2 mL of acid phosphatase solution with a final concentration of 5 U / L to 1.45 mL of acetate buffer (50 mmol / L), mixed well and placed in a 37°C water bath for 30 min. After the end, 0.15 mL of the gold nanocluster solution prepared in Example 1 was mixed with the above reaction solution, and then the fluorescence emission intensity value of the solution at 590 nm (F 590 ) (excitation wavelength is 425 nm), each group was measured three times in parallel. A control group (without acid phosphatase) was set under each pH condition. According to the fluorescence emission intensity F between the experimental group and the control group 590 The difference (△F) to judge the optimal reaction conditions of the reaction system. Depend on figure 2 It can be seen that the optimal reaction pH of the acid phosphatase assay system is 5.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com