A kind of biotin-labeled naringin, preparation method and application thereof
A technology of biotin labeling and naringin, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of unclear drug targets of naringin, achieve good anti-inflammatory effect, and simple preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of the naringin of embodiment 1 biotinylation
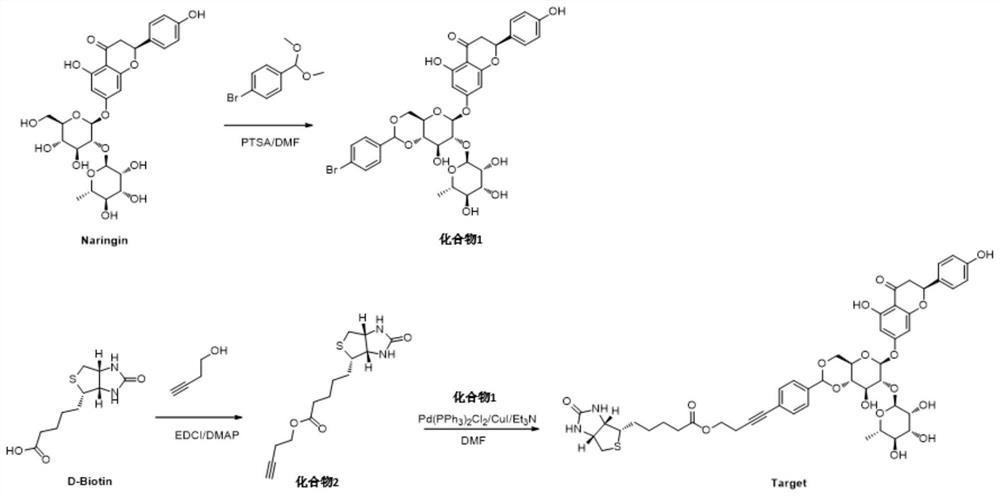
[0038](1) Add 2.6g of naringin, 30mg of 4-toluenesulfonamide (PTSA) and 15mL of organic solvent N,N-dimethylformamide DMF into a round bottom flask, and stir to dissolve it. Add 2.6 g of p-bromobenzaldehyde dimethyl acetal, and react at 35°C for 12-24 hours, 20 hours in this example. In this step of other embodiments, the amount of each substance can meet the following conditions: the molar ratio of naringin, PTSA and p-bromobenzaldehyde dimethyl acetal is 25-45:1:50-80; naringin and organic solvent The mass-to-volume ratio of naringin is 1:5-10, that is, the volume of organic solvent per gram of naringin is 5-10 milliliters.
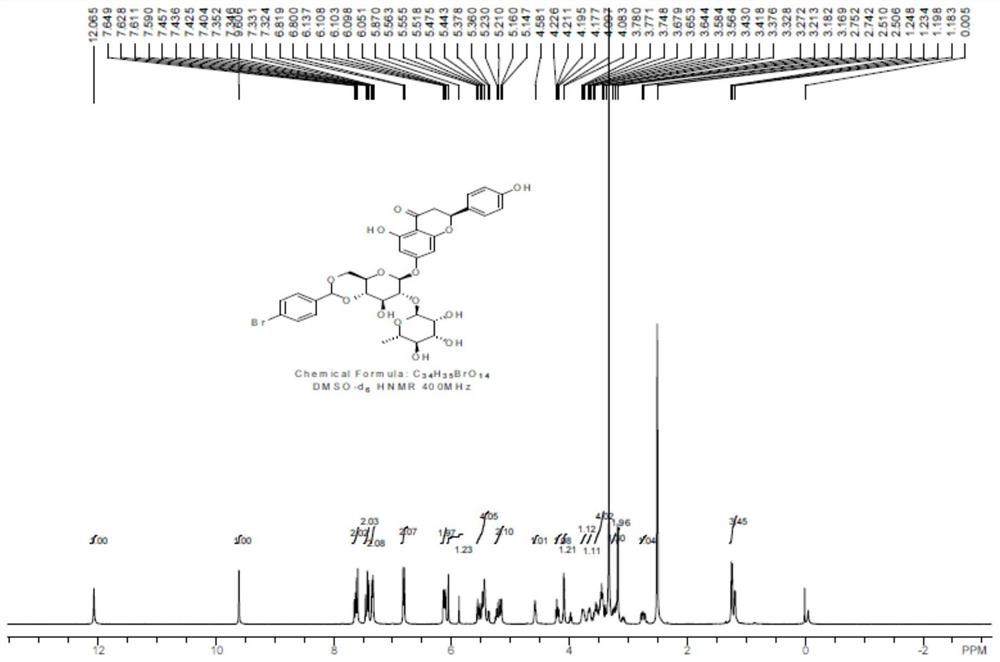
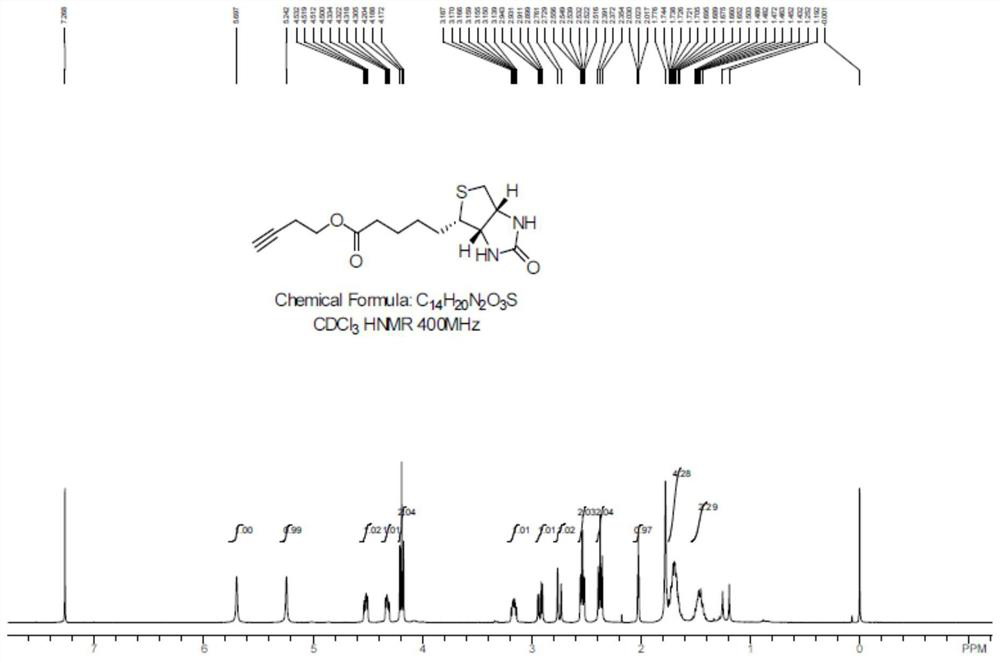
[0039] After the reaction, DMF was distilled off under reduced pressure, and the residue was recrystallized from methanol to obtain compound 1 as a white solid with a yield of 40%. Carry out NMR analysis to the obtained white solid compound 1, 1 H NMR spectrum see figure 2 , the...
Embodiment 2
[0049] Example 2 Biotin-labeled naringin anti-inflammatory pharmacodynamics study
[0050] To test whether the substance still has anti-inflammatory activity after biotin-labeled naringin. Inflammation model of mouse macrophage RAW264.7 cells was established with lipopolysaccharide (LPS). RAW264.7 cells in the logarithmic growth phase were seeded in 24-well culture plates, and after one day of adherent growth, four different treatments were set, namely, the blank control group of RPMI-1640 medium without fetal bovine serum ; lipopolysaccharide LPS (1 μg / mL) treatment group; 50 μg / mL biotin-labeled naringin probe (Biotin-Naringin) and lipopolysaccharide LPS (1 μg / mL) treatment group; and naringin (50 μg / mL ) and lipopolysaccharide LPS (1 μg / mL). After 18 hours of drug treatment, remove the cell supernatant, add Trizol reagent, extract RNA according to the operation of the kit, and after reverse transcription, use real-time fluorescent quantitative PCR to detect the inflammato...
Embodiment 3
[0071] Example 3 Immunoprecipitation Screening for Binding Proteins of Naringin
[0072] In order to screen out the target protein specifically binding to naringin from mouse macrophages, the synthesized biotin-labeled naringin was used as a probe, and the method of immunoprecipitation was used for screening.
[0073] The procedure of immunoprecipitation assay is as follows: Figure 7shown. Take 60 μL of streptavidin agarose beads respectively in two 1.5 mL centrifuge tubes, add 500 μL of phosphate buffer saline (PBS) to each, and centrifuge to remove the supernatant (14000 g, 5 s). Add 500 μL of PBS and 4 μL of 1 μg / μL Biotin-Naringin and Biotin solutions respectively, and incubate at 4° C. for 1 h with shaking. After the incubation, centrifuge to remove the supernatant (14000g, 5s). Add 500 μL PBS and centrifuge and wash 3 times (14000g, 5s). Then add 500 μL of 1 μg / μL mouse macrophage total protein, and incubate at 4°C for 1 h with shaking. After the incubation, centri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com