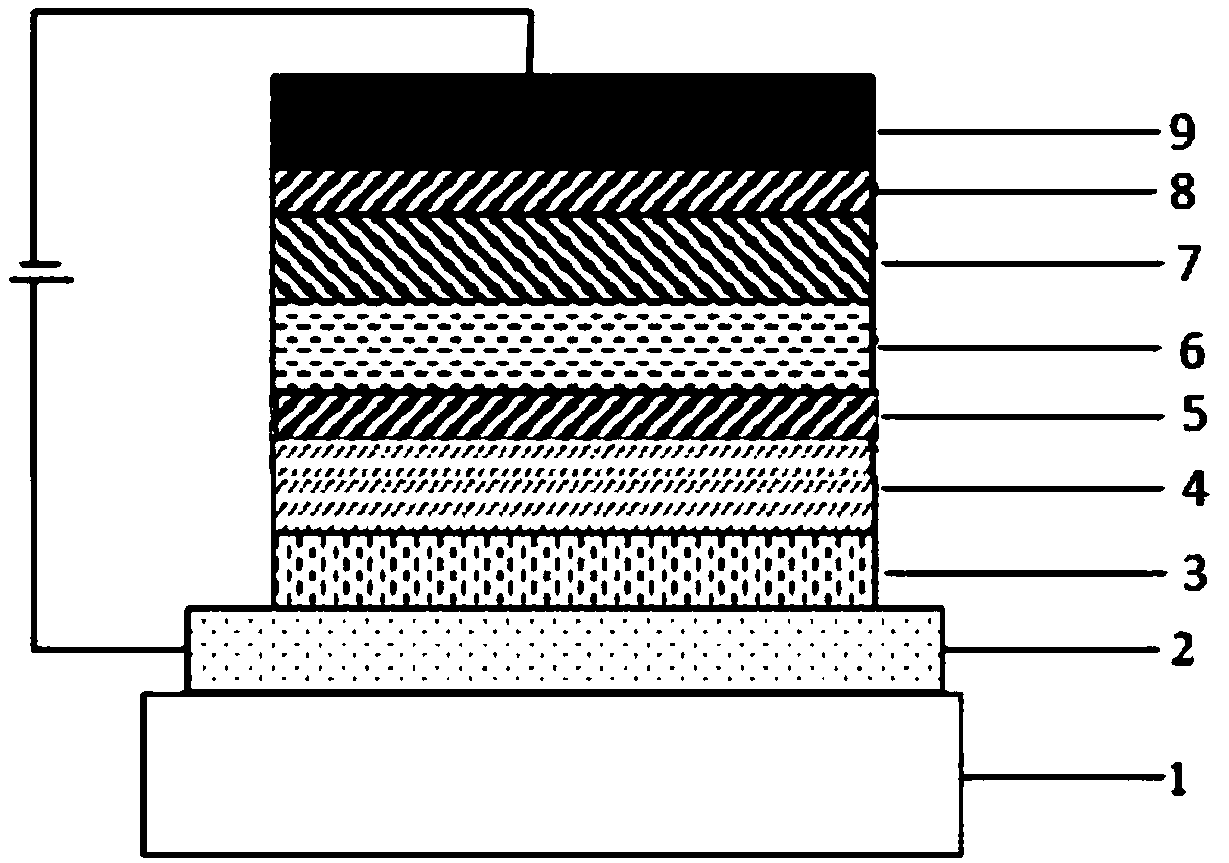
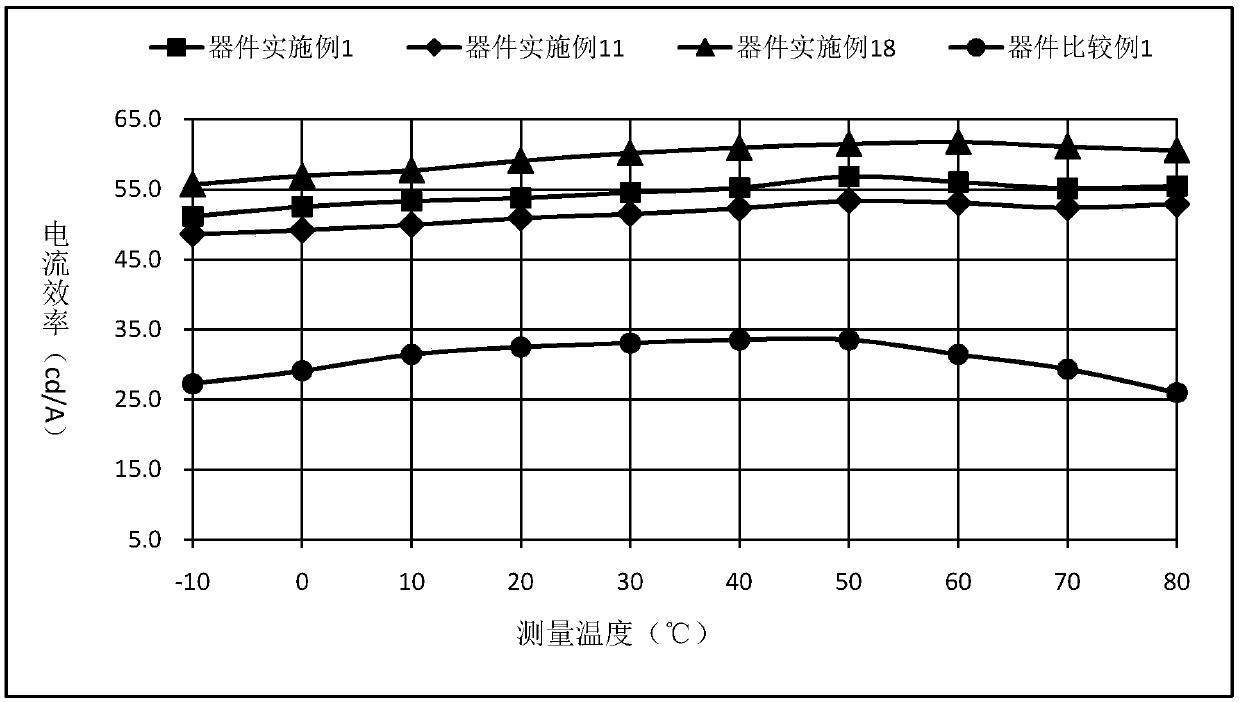
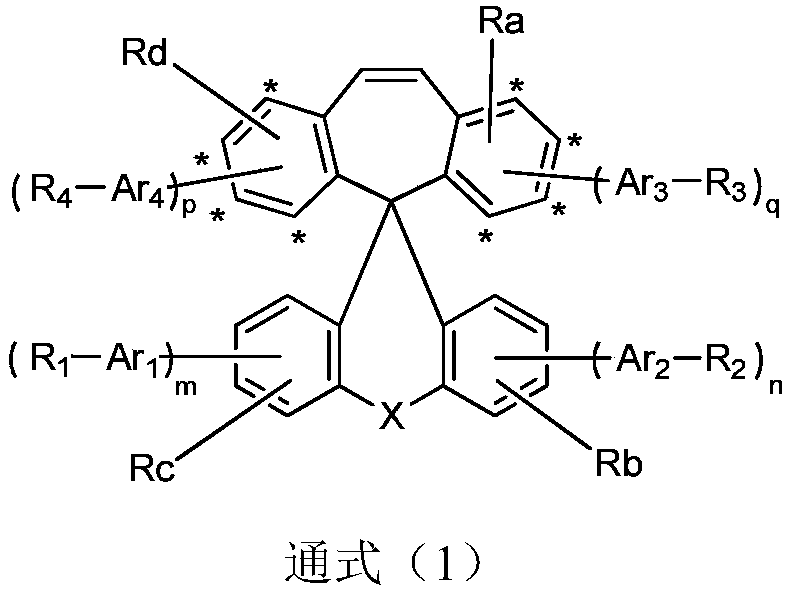
Compound using spirobibenzocycloheptene fluorene as core, and applications in organic electroluminescent devices
A technology of benzocycloheptene fluorene and compound, applied in the application field of organic electroluminescence devices, can solve problems such as disparity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1: the synthesis of intermediate AI:
[0082]
[0083] (1) Weigh the raw material H1 and dissolve it in tetrahydrofuran, then add the raw material L1, react the above reactant mixed solution at 60°C for 24 hours, add saturated NHCl 4 Grignard salt is converted into alcohol; after the reaction is completed, ether extraction, drying and rotary evaporation, petroleum ether: dichloromethane mixed solvent (3:2) silica gel column purification to obtain intermediate F1; the molar ratio of the raw material H1 to the raw material L1 It is 1:1.0~1.5.
[0084] (2) Weigh intermediate F1, dissolve it with dichloromethane, add boron trifluoride etherate complex dropwise at room temperature, react for 30 minutes, add ethanol and water to quench the reaction, extract with dichloromethane, dry and spin Steam, petroleum ether silica gel column purification, recrystallization with ethanol: dichloromethane (volume ratio 1:1), obtain intermediate F2; The mol ratio of describe...
Embodiment 2
[0096] Embodiment 2: the synthesis of intermediate AII:
[0097]
[0098] (1) Under the protection of nitrogen, weigh the raw material H2, stir and dissolve it with tetrahydrofuran, add the raw material L2 dropwise, react at 60°C for 24 hours, a large amount of white precipitate is formed, and finally add saturated NHCl 4 Convert the Grignard salt into alcohol; after the reaction, extract with ether, dry and rotary evaporate, and purify on a silica gel column with a mixed solvent of petroleum ether: dichloromethane (3:2) to obtain a slightly yellow solid intermediate G1. The molar ratio of the raw material H2 to the raw material L1 is 1:1.0-1.5.
[0099] (2) Weigh intermediate G1, dissolve in dichloromethane, add boron trifluoride etherate complex dropwise at room temperature, react for 30 minutes, add ethanol and water to quench the reaction, extract with dichloromethane, dry and spin Evaporation, petroleum ether silica gel column purification, and recrystallization with ...
Embodiment 3
[0117] Embodiment 3: the synthesis of intermediate A15:
[0118]
[0119] (1) In a 500mL three-neck flask, under nitrogen protection, add 0.05mol raw material H3-1, 0.06mol raw material Q2-1, dissolve with a mixed solvent (180ml toluene, 90ml ethanol), and then add 0.15mol Na 2 CO 3 aqueous solution (2M), stirred under nitrogen for 1 hour, then added 0.0005mol Pd(PPh 3 ) 4 , heating to reflux for 15 hours, sampling point plate, the reaction is complete. Naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain an intermediate M1-1 with a HPLC purity of 98.4% and a yield of 76.1%. Elemental analysis structure (molecular formula C 21 h 14 o 3 ): theoretical value C, 80.24; H, 4.49; 0, 15.27; test value: C, 80.24; H, 4.50; 0, 15.26. ESI-MS(m / z)(M + ): The theoretical value is 314.09, and the measured value is 314.02.
[0120] (2) In a 250mL three-necked flask, under the protection of nitrogen, add 0.03mol of inter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Material molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com