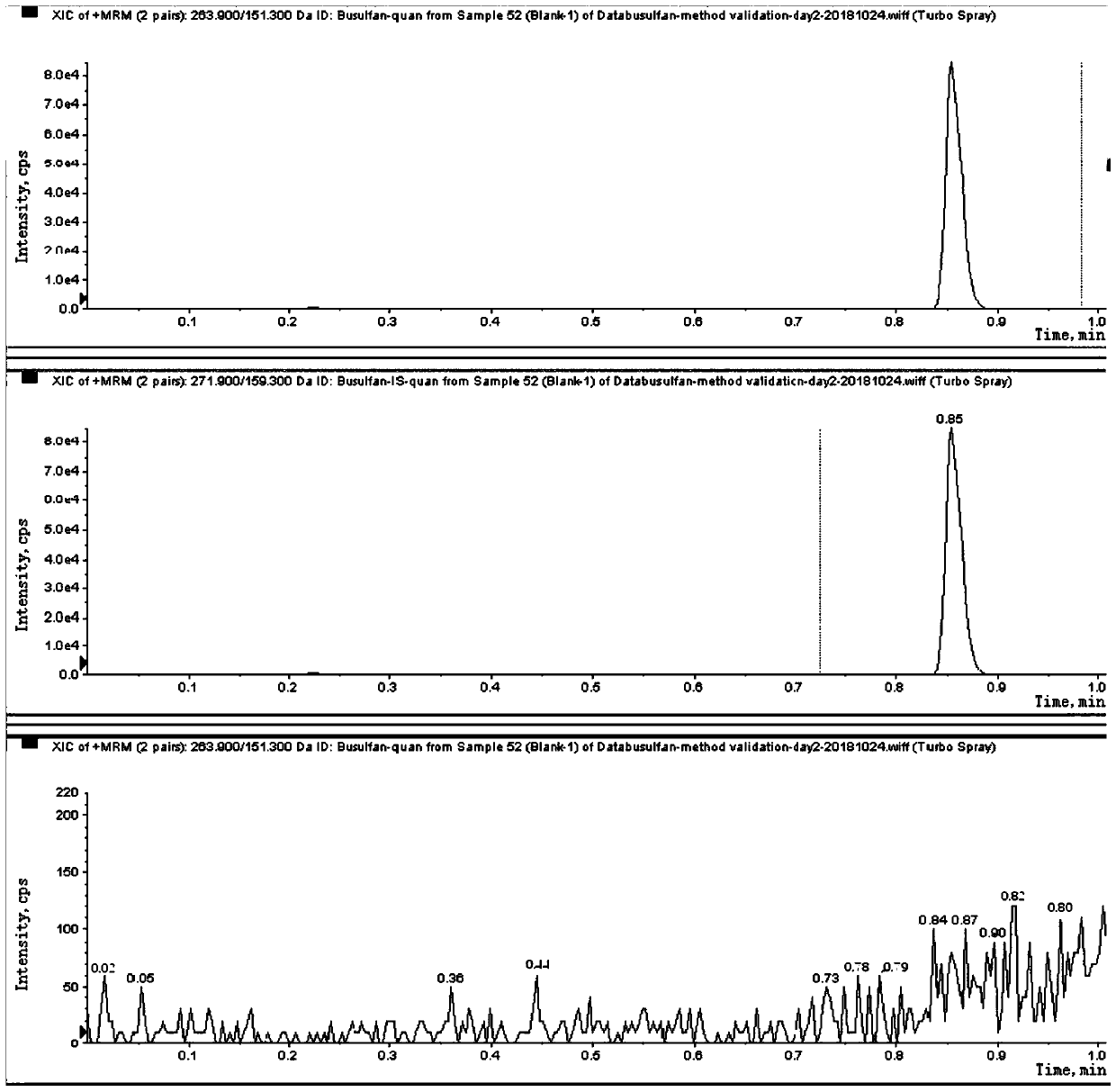
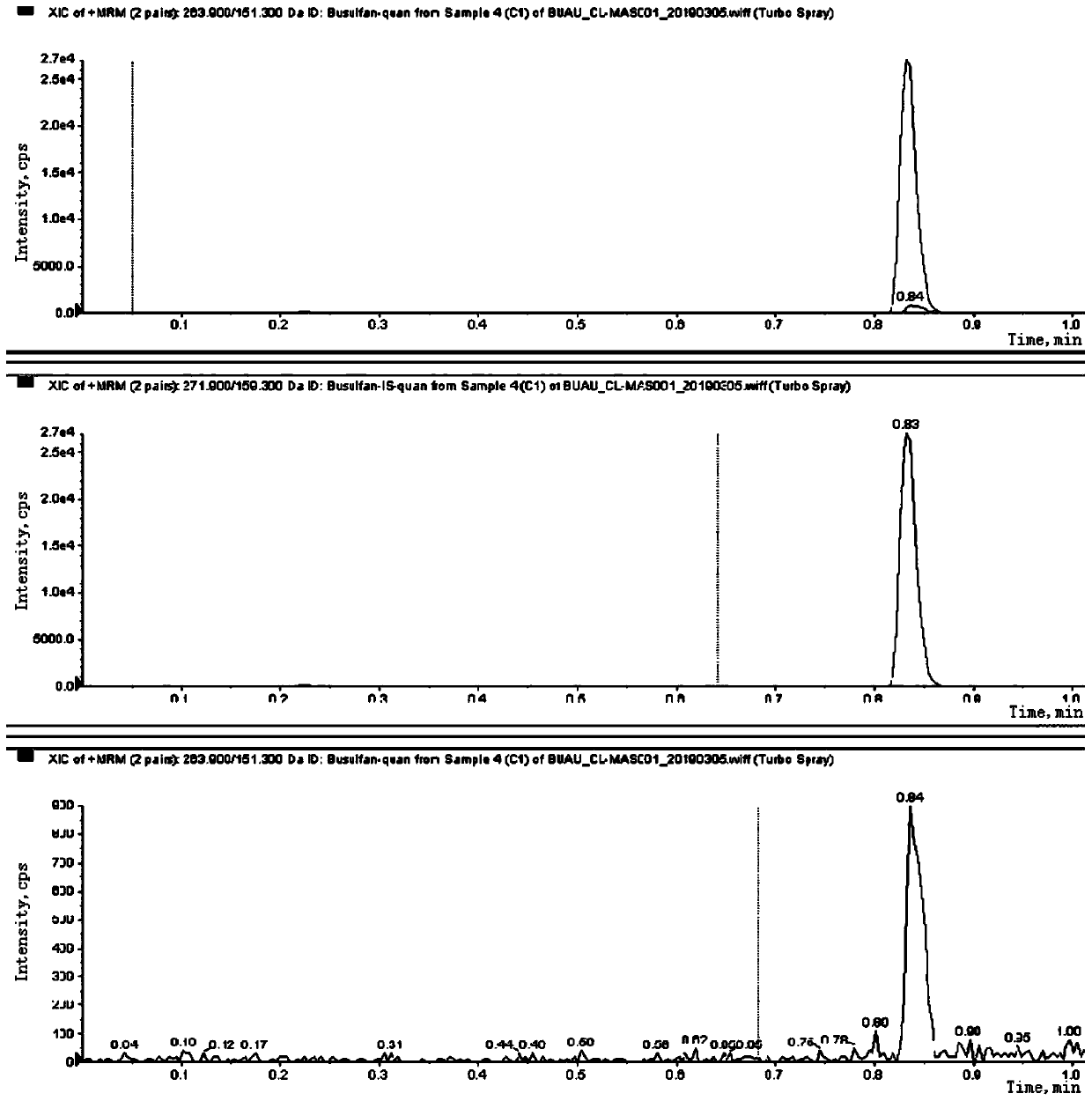
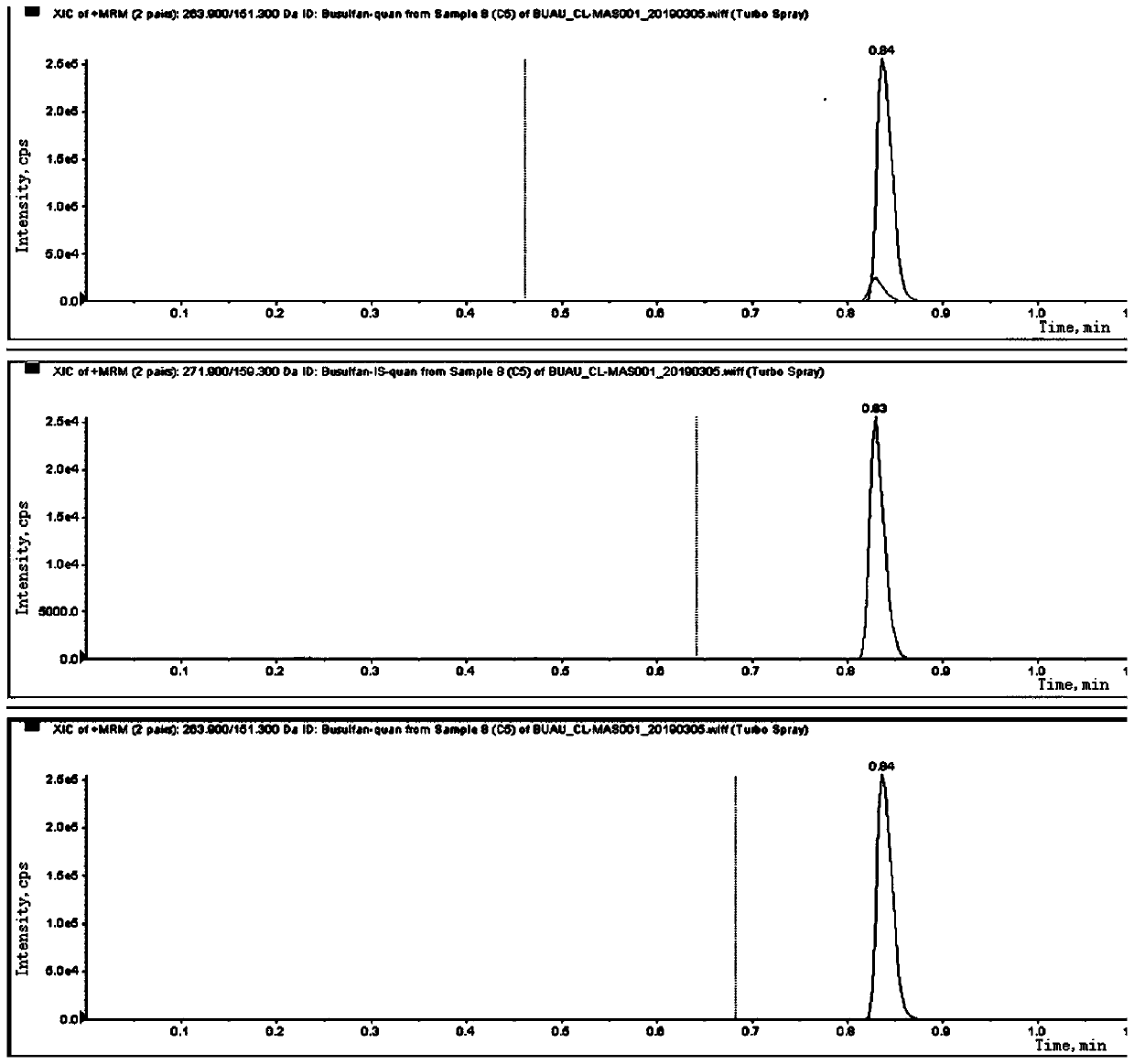
Liquid chromatography tandem mass spectrometry (LC-MS/MS) method for detecting busulfan in plasma
A technology of liquid chromatography and tandem mass spectrometry, which is applied in the field of detection of busulfan in plasma by liquid chromatography and tandem mass spectrometry, which can solve the problems of long instrument analysis time, long method processing time, and insufficient analysis sensitivity, so as to save detection time , high precision, and shortened analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment one: specific implementation steps of the present invention
[0044] 1. Preparation of standard / quality control primary stock solution
[0045] 1.1 Standard primary stock solution (SSC): Busulfan standard methanol solution (Cerilliant) with a concentration of 1mg / mL, numbered as SSC, stored at -25~-15°C until the expiration date of the manufacturer;
[0046] 1.2 Quality control primary stock solution (SSQC): Weigh 10 mg of busulfan (TRC / Sigma) quality control with a million-level analytical balance, transfer to a 10 mL volumetric flask, and dilute to 10 mL with pure methanol to prepare The primary stock solution of the quality control product with a concentration of 1 mg / mL was shaken, mixed and ultrasonically transferred to a brown glass vial, numbered SSQC, and stored at -25 to -15°C with a validity period of one year.
[0047] 2. Preparation of secondary stock solution for standard / quality control
[0048] 2.1 Standard secondary stock solution (sub-SSC...
Embodiment 2
[0094] Example 2: Methodological verification
[0095] 1. Precision
[0096] Add corresponding amount of busulfan standard substance to blank matrix (100% bovine plasma heparin sodium anticoagulant), and prepare three concentration levels of busulfan in the linear range (25-7500ng / mL) of the calibration curve. The concentrations of Xiaoan standard solution are: 300ng / mL, 2500ng / mL, and 5000ng / mL. There are 3 parallel samples for each concentration level, which is a batch; a total of five batches are divided into 5 days for verification. a batch.
[0097] Table 5 precision data results
[0098] Busulfan Low concentration medium concentration High concentration Intra-assay precision (CV,%) 5.01 2.37 1.20 Inter-assay precision (CV,%) 3.43 3.79 3.04
[0099] The verification results are shown in Table 5. The CV% of the intra-assay and inter-assay precision is within 15%, which meets the verification requirements; and the CV% of the intra-as...
Embodiment 3
[0144] Example 3: Patient sample monitoring case
[0145] Case 1:
[0146] Basic information: Male, aged 47 years, weighing 75kg, administered intravenous dose of busulfan 120mg, completed venous blood collection 2 hours after intravenous drug injection, and 1, 4, 6, 8 hours after the first blood collection respectively Venous blood was collected, and the plasma was centrifuged as soon as possible and anticoagulated with sodium heparin. After performing sample pretreatment on the machine for sample analysis, a drug-time curve is established, such as Figure 6 shown.
[0147] Case 2:
[0148] Basic information: female, age 32, body weight 67.2kg, intravenous dose of busulfan 108mg, venous blood collection completed 2 hours after intravenous drug injection, and 1, 4, 6, 8 days after the first blood collection respectively Venous blood was collected every hour, and the plasma was centrifuged as soon as possible and added heparin sodium for anticoagulation. After performing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com