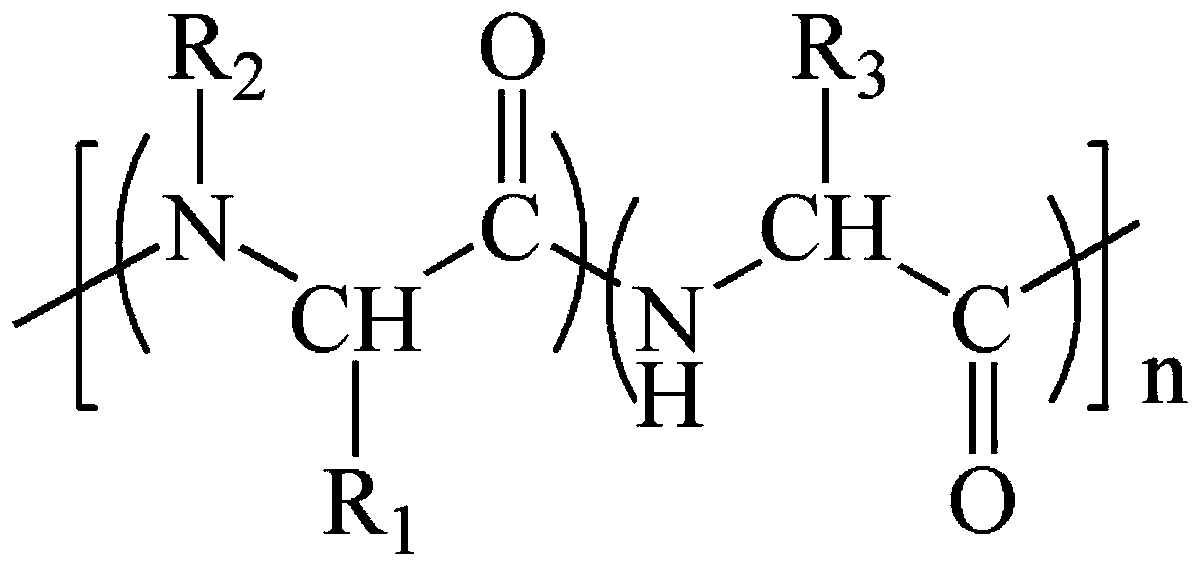
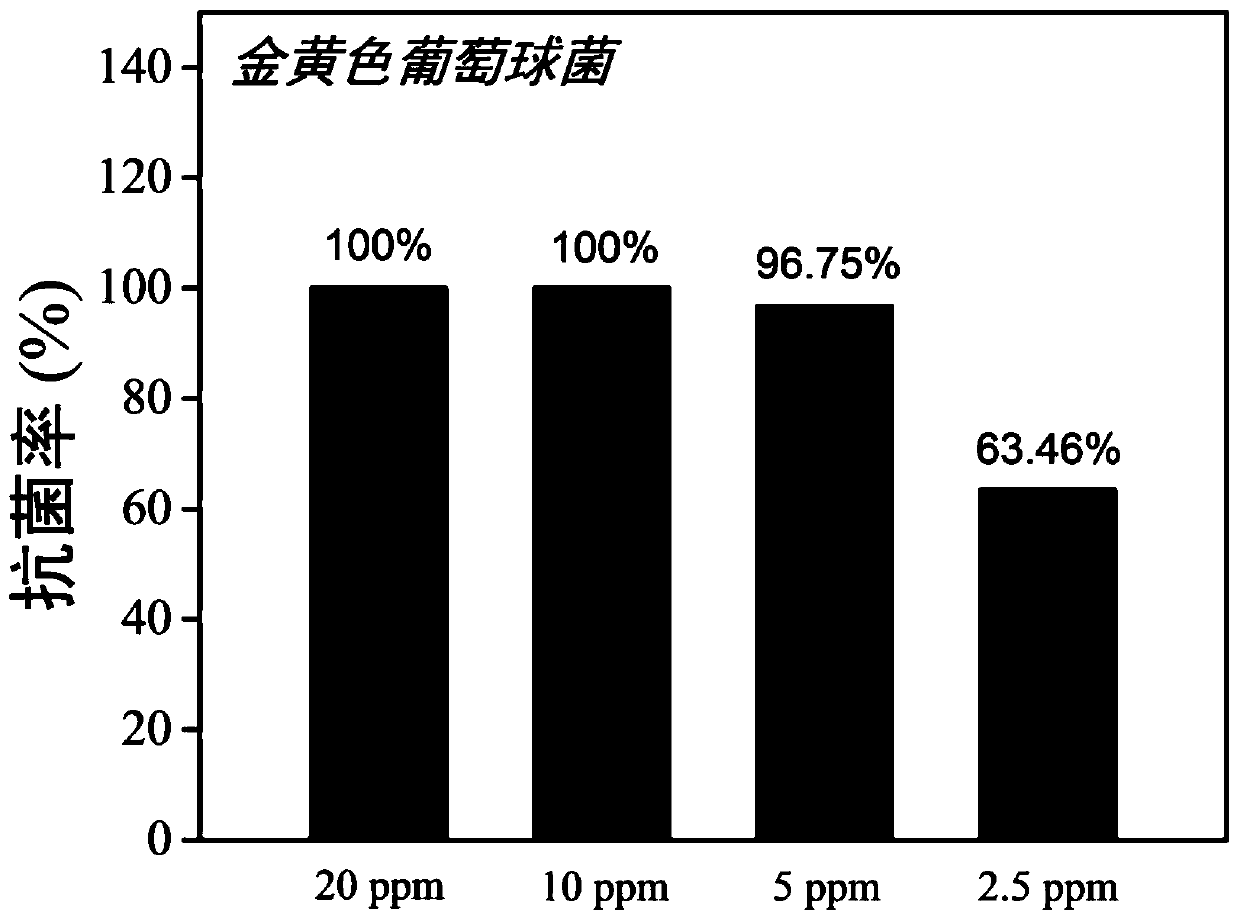
Antibacterial polyamino acid derivatives or copolymers having alternating structures and preparation method of antibacterial polyamino acid derivatives or copolymers
A polyamino acid, alternating structure technology, applied in the fields of botanical equipment and methods, chemicals for biological control, biocides, etc., can solve the problem of inability to effectively control the hydrophilicity and hydrophobicity of polymers, the degradation rate of material strength, antibacterial activity and biosafety problems, such as the inability to precisely adjust the structure of polyamino acid copolymers, and exploration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] This embodiment prepares a kind of alternate structure degradable antibacterial polyamino acid copolymer
[0071] 1) Polymerization reaction: Add 0.01moL of 5-(benzyloxycarbonylamino)pentanal and 0.01moL of benzylamine in sequence to 10mL of tetrahydrofuran for reaction at room temperature. After stirring for 3 days, the solvent was evaporated from the solution under reduced pressure to obtain a yellow oily intermediate. Dissolve 0.01 mol of potassium isocyanate in 5 mL of methanol at 0°C, and add 0.01 mol of hydrofluoric acid and the yellow oily intermediate in sequence. The above mixture was warmed to room temperature and stopped after stirring for 4 days. Add distilled water, extract the purified water layer, and obtain amino acid derivatives with protective groups.
[0072] 2) Deprotection group reaction: dissolve the polyamino acid derivative containing benzyloxycarbonyl protecting group in 20 mL of trifluoroacetic acid, add 5 mL of 33% hydrobromic acid in acetic...
Embodiment 2
[0083] This embodiment prepares a kind of alternate structure degradable antibacterial polyamino acid copolymer
[0084] 1) Polymerization reaction: Add 0.01moL 4-methoxybenzylamine, 0.01moL methanesulfonic acid, 0.01moL potassium isocyanate, 0.01moL 4-(benzyloxycarbonylamino)butyraldehyde to 5mL tetrahydrofuran in sequence at 0°C , then the mixture was heated to room temperature, stirred for 15 days, the reaction was stopped, distilled water was added, and the purified water layer was extracted to obtain amino acid derivatives with protective groups.
[0085] 2) Deprotection reaction: dissolve the polyamino acid derivative containing benzyloxycarbonyl protecting group in 5 mL of methanesulfonic acid, react at 50°C for 3 days, the product is precipitated with methyl tert-butyl ether, washed three times with ethanol, and dialyzed at 1000 molecular weight The bag was dialyzed for 3 days to obtain polyamino acid derivatives with deprotected groups.
[0086] The reaction scheme i...
Embodiment 3
[0090] This embodiment prepares a kind of alternate structure degradable antibacterial polyamino acid copolymer
[0091] 1) Polymerization reaction: Add 0.01moL 4-methoxybenzylamine, 0.01moL methanesulfonic acid, 0.01moL potassium isocyanate, 0.01moL 4-(trifluoroacetylamino)butyraldehyde to 5mL in sequence at 0°C Then heat the mixture to room temperature, stir for 15 days, stop the reaction, add distilled water, dry the organic layer, remove water, evaporate the solvent, dry the obtained product, and purify to obtain an amino acid derivative with a protecting group.
[0092] 2) Deprotection group reaction: dissolve polyamino acid derivatives containing trifluoroacetyl group protection groups in 40 mL of 1mol / L sodium hydroxide solution, react at 50°C for 5 hours, and dialyze the product for 3 days with a 1000 molecular weight dialysis bag to obtain Deprotected polyamino acid derivatives.
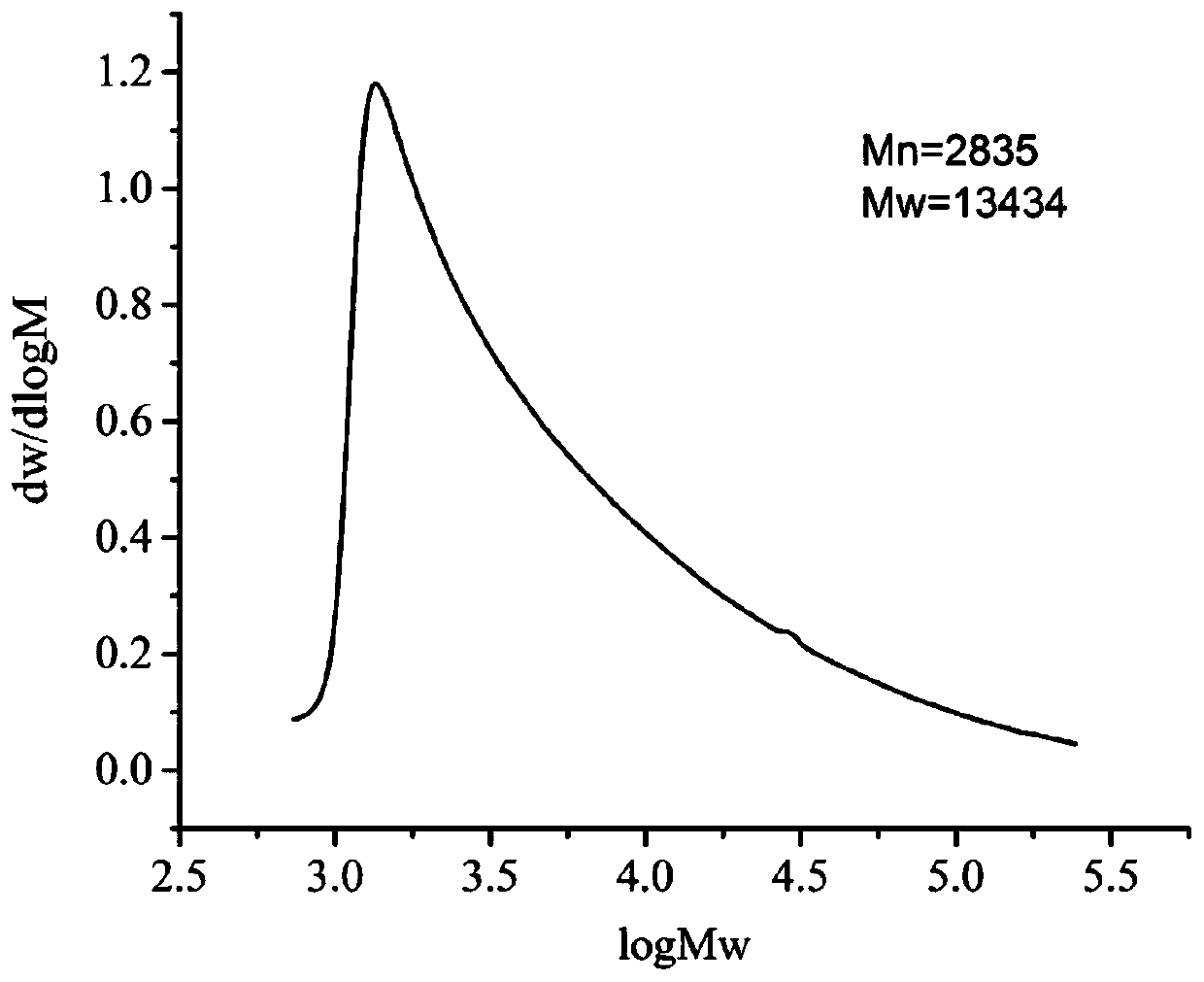
[0093] The number-average molecular weight of the polyamino acid derivatives that this ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com